
Recent Developments on the Mechanism and Kinetics
... Krause et al., 2009; Martínez et al., 2011). The vast majority of esters can be prepared using esterification reaction in the chemical engineering industry. Esterification has acquired further improvement from the engineering side; this mainly depends on the research of esterification kinetics. On ...
... Krause et al., 2009; Martínez et al., 2011). The vast majority of esters can be prepared using esterification reaction in the chemical engineering industry. Esterification has acquired further improvement from the engineering side; this mainly depends on the research of esterification kinetics. On ...
13AP General Equilibrium FR worksheet (missing 1988)
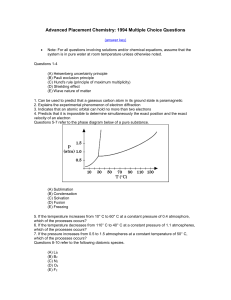
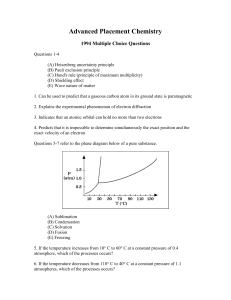
... atmospheres when equilibrium was reached. Calculate the number of moles of H2O(g) present at equilibrium. (b) How many grams of the original solid remain in the container under the conditions described in (a)? (c) Write the equilibrium expression for the equilibrium constant, Kp, and calculate its v ...
... atmospheres when equilibrium was reached. Calculate the number of moles of H2O(g) present at equilibrium. (b) How many grams of the original solid remain in the container under the conditions described in (a)? (c) Write the equilibrium expression for the equilibrium constant, Kp, and calculate its v ...
Page 1
... 57. Compare fission and fusion. (define and identify uses) Fission: the splitting of a nucleus into fragments Fusion: the combining of atomic nuclei 58. Compare alpha, beta and gamma particles. Alpha: a particle with twp protons and two neutrons, with a 2+ charge; is equivalent to a helium -4 nucleu ...
... 57. Compare fission and fusion. (define and identify uses) Fission: the splitting of a nucleus into fragments Fusion: the combining of atomic nuclei 58. Compare alpha, beta and gamma particles. Alpha: a particle with twp protons and two neutrons, with a 2+ charge; is equivalent to a helium -4 nucleu ...
Experimental skills and abilities
... used, the separation may be greater or less depending on how the dyes dissolve in the new solvent. 2 The substances to be separated do not have to be coloured. For example, amino acids obtained by hydrolysis of proteins are colourless. Colourless substances can be made visible by spraying the chrom ...
... used, the separation may be greater or less depending on how the dyes dissolve in the new solvent. 2 The substances to be separated do not have to be coloured. For example, amino acids obtained by hydrolysis of proteins are colourless. Colourless substances can be made visible by spraying the chrom ...
L-12 Spontaneity of chemical reactions
... constant. But it does not tell us whether a specified change or a process including a chemical reaction can occur spontaneously i.e., whether it is feasible or not. For example, the first law does not deny the possibility that a metal bar having a uniform temperature can spontaneously become warmer ...
... constant. But it does not tell us whether a specified change or a process including a chemical reaction can occur spontaneously i.e., whether it is feasible or not. For example, the first law does not deny the possibility that a metal bar having a uniform temperature can spontaneously become warmer ...
MC94 - Southchemistry.com
... 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the ...
... 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... By carrying out a series of displacement reactions, which follow the pattern shown below, between metal atoms and metal ions, a series of reactivity can be deduced with the strongest reducing agent at the top as the most reactive metal. XCl (aq) + Y (s) NaY (aq) + X If the reaction above is feasi ...
... By carrying out a series of displacement reactions, which follow the pattern shown below, between metal atoms and metal ions, a series of reactivity can be deduced with the strongest reducing agent at the top as the most reactive metal. XCl (aq) + Y (s) NaY (aq) + X If the reaction above is feasi ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... By carrying out a series of displacement reactions, which follow the pattern shown below, between metal atoms and metal ions, a series of reactivity can be deduced with the strongest reducing agent at the top as the most reactive metal. XCl (aq) + Y (s) NaY (aq) + X If the reaction above is feasi ...
... By carrying out a series of displacement reactions, which follow the pattern shown below, between metal atoms and metal ions, a series of reactivity can be deduced with the strongest reducing agent at the top as the most reactive metal. XCl (aq) + Y (s) NaY (aq) + X If the reaction above is feasi ...
Kinetic modelling of the Maillard reaction between proteins and sugars
... 1.3 Sugar degradation ...
... 1.3 Sugar degradation ...
Chemical Reactions and The Mole
... does all that mass or stuff go? It is converted into another form. These new forms are gasses. There is a lot of carbon, oxygen and hydrogen in living organisms. When a living organism burns, these atoms will now be found in the following molecules H2O and CO2. The remaining soot is mostly carbon, p ...
... does all that mass or stuff go? It is converted into another form. These new forms are gasses. There is a lot of carbon, oxygen and hydrogen in living organisms. When a living organism burns, these atoms will now be found in the following molecules H2O and CO2. The remaining soot is mostly carbon, p ...
chemistry-c7-what-you-should
... I can recall that the feedstocks of nitrogen and hydrogen for the Haber process are made from air, natural gas and steam I in the context of the Haber process: a. I understand that the reaction between hydrogen and nitrogen to form ammonia is a reversible reaction b. I understand how the yield of am ...
... I can recall that the feedstocks of nitrogen and hydrogen for the Haber process are made from air, natural gas and steam I in the context of the Haber process: a. I understand that the reaction between hydrogen and nitrogen to form ammonia is a reversible reaction b. I understand how the yield of am ...
Advanced Placement Chemistry
... 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the ...
... 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the ...
17 ADSORPTION AND CATALYSIS S MODULE - 5
... cubes, each of side equal to 1 10 –6 cm the surface area will increase to 6 106 cm2 or 600 m2. The increase in surface area would result in greater adsorption. ...
... cubes, each of side equal to 1 10 –6 cm the surface area will increase to 6 106 cm2 or 600 m2. The increase in surface area would result in greater adsorption. ...
A “Tag-and-Modify” Approach to Site
... ovalent modification can expand a protein's functional capacity. Fluorescent or radioactive labeling, for instance, allows imaging of a protein in real time. Labeling with an affinity probe enables isolation of target proteins and other interacting molecules. At the other end of this functional spec ...
... ovalent modification can expand a protein's functional capacity. Fluorescent or radioactive labeling, for instance, allows imaging of a protein in real time. Labeling with an affinity probe enables isolation of target proteins and other interacting molecules. At the other end of this functional spec ...
Homework Booklet [4,S]
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity when ...
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity when ...
Balancing Reaction Equations Oxidation State Reduction
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
... Balance each of these two reactions using the eight steps we discussed. Assume that the reactions take place in alkaline solution. ...
IGCSE SoW 2013
... Understand that the breaking of bonds is endothermic and that the making of bonds is exothermic ...
... Understand that the breaking of bonds is endothermic and that the making of bonds is exothermic ...
mc_ch08 - MrBrownsChem1LCHS
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
Stoichiometry Notes
... Calculate the normality of each of the following solutions : (a) 7.88 g of HNO3 per L solution (b) 26.5 g of Na2CO3 per L solution (if acidified to form CO2). [At. wt. C = 12, N = 14, O = 16, Na = 23] (a) What volume of 5.00 N H2SO4 is required to neutralize a solution containing 2.50 g NaOH ? (b) H ...
... Calculate the normality of each of the following solutions : (a) 7.88 g of HNO3 per L solution (b) 26.5 g of Na2CO3 per L solution (if acidified to form CO2). [At. wt. C = 12, N = 14, O = 16, Na = 23] (a) What volume of 5.00 N H2SO4 is required to neutralize a solution containing 2.50 g NaOH ? (b) H ...
IChO_Comp_Prob_Answ 1997
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
29th INTERNATIONAL CHEMISTRY OLYMPIAD PREPARATORY
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...














![Homework Booklet [4,S]](http://s1.studyres.com/store/data/010355871_1-63c750e3d1b58eaaebbb3f5d45651c44-300x300.png)






