Tests for functional groups
... b) Ketones (R2CO) Ketones are not reducing agents; they may not be oxidised to carboxylic acids. If a carbonyl compound gives negative results with any of the tests above it confirms the presence of a ketone. i) Methyl ketones (RCOCH3) – the iodoform (triiodomethane) reaction Methyl ketones undergo ...
... b) Ketones (R2CO) Ketones are not reducing agents; they may not be oxidised to carboxylic acids. If a carbonyl compound gives negative results with any of the tests above it confirms the presence of a ketone. i) Methyl ketones (RCOCH3) – the iodoform (triiodomethane) reaction Methyl ketones undergo ...
Chemical Reactions Chemical Arithmetic
... ratios to convert known information into required information • 3) Set up factor-label expression and calculate the answer • 4) Estimate or check answer using a ballpark solution ...
... ratios to convert known information into required information • 3) Set up factor-label expression and calculate the answer • 4) Estimate or check answer using a ballpark solution ...
kinetics and equilibrium
... • Works by adsorption – the adherence of one substance to the surface of another • Catalyst has specific lumps that hold the chemicals in the right position to react (increase the chance of them ...
... • Works by adsorption – the adherence of one substance to the surface of another • Catalyst has specific lumps that hold the chemicals in the right position to react (increase the chance of them ...
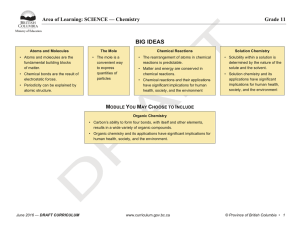
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Demonstrate an awareness of assumptions, question information given, and identify bias in their own work and in primary and secondary sources • Consider the changes in knowledge over time as tools and technologies have developed • Connect scientific explorations to careers in science • Exercise a ...
... • Demonstrate an awareness of assumptions, question information given, and identify bias in their own work and in primary and secondary sources • Consider the changes in knowledge over time as tools and technologies have developed • Connect scientific explorations to careers in science • Exercise a ...
MULTIPLE CHOICE. Choose the one alternative that best completes
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...
... 11) Ionic bonds are formed when A) atoms share electrons. B) two or more atoms lose electrons at the same time. C) electrons are completely transferred from one atom to another. D) hydrogen forms bonds with negatively charged atoms in the same or different molecule. E) a pair of electrons is shared ...
AP® Chemistry 2009 Free-Response Questions - AP Central
... (e) Calculate the percent error in the value of the molar mass calculated in part (d). (f) For each of the following two possible occurrences, indicate whether it by itself could have been responsible for the error in the student’s experimental result. You need not include any calculations with your ...
... (e) Calculate the percent error in the value of the molar mass calculated in part (d). (f) For each of the following two possible occurrences, indicate whether it by itself could have been responsible for the error in the student’s experimental result. You need not include any calculations with your ...
Chemical reactions revision
... oxygen. ‘Oxygen’ does not show up in the name; the ‘ate’ is the only clue it is there You should be able to give the name of the compound formed when different elements combine and tell which elements are present in any simple compound ...
... oxygen. ‘Oxygen’ does not show up in the name; the ‘ate’ is the only clue it is there You should be able to give the name of the compound formed when different elements combine and tell which elements are present in any simple compound ...
Ch.08An Introduction to Metabolism
... (b) Mechanical work: ATP binds noncovalently to motor proteins, then is hydrolyzed ...
... (b) Mechanical work: ATP binds noncovalently to motor proteins, then is hydrolyzed ...
Chemical Reactions
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
... – Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken – Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. – Symbols represent elements, formulas describe compounds, chemical equations describe ...
SOL Essential Knowledge
... 2. Ionization energy is the energy required to remove the most easily held electron. 3. Elements with low ionization energy form ions easily. F. Recognize that transition metals can have multiple oxidation states. G. Summarize the following concepts about covalent bonding: 1. Covalent bonds involve ...
... 2. Ionization energy is the energy required to remove the most easily held electron. 3. Elements with low ionization energy form ions easily. F. Recognize that transition metals can have multiple oxidation states. G. Summarize the following concepts about covalent bonding: 1. Covalent bonds involve ...
Sample % Sulfate Absolute Deviation A 44.02 B 44.11 C 43.98 D
... C. WORKSHEET 3…EXPLORING FUNDAMENTAL CHEMICAL LAWS ...
... C. WORKSHEET 3…EXPLORING FUNDAMENTAL CHEMICAL LAWS ...
AQA_GCSE_Chemistry_Higher_Unit_2_Notes
... when reacting limestone with hydrochloric acid, different concentrations of hydrochloric acid could be used and the time taken to collect a boiling-tube of gas (50cm3) could be ...
... when reacting limestone with hydrochloric acid, different concentrations of hydrochloric acid could be used and the time taken to collect a boiling-tube of gas (50cm3) could be ...
in a Chemical Reactor - Max-Planck
... wire cushions for these reactions and make the whole process a form of reactive distillation. We are currently working to identify the best conditions for obtaining cyclohexanol in this way. Here, too, we are faced with the problem that cyclohexene and formic acid are not particularly mutually solub ...
... wire cushions for these reactions and make the whole process a form of reactive distillation. We are currently working to identify the best conditions for obtaining cyclohexanol in this way. Here, too, we are faced with the problem that cyclohexene and formic acid are not particularly mutually solub ...
2002 AP Chemistry Free-Response Questions
... 2002 AP® CHEMISTRY FREE-RESPONSE QUESTIONS 3. Consider the hydrocarbon pentane, C5H12 (molar mass 72.15 g). (a) Write the balanced equation for the combustion of pentane to yield carbon dioxide and water. (b) What volume of dry carbon dioxide, measured at 25C and 785 mm Hg, will result from the co ...
... 2002 AP® CHEMISTRY FREE-RESPONSE QUESTIONS 3. Consider the hydrocarbon pentane, C5H12 (molar mass 72.15 g). (a) Write the balanced equation for the combustion of pentane to yield carbon dioxide and water. (b) What volume of dry carbon dioxide, measured at 25C and 785 mm Hg, will result from the co ...
printable version
... same formula as Kc but means that the reaction is not necessarily at equilibrium. (Instantaneous) • If Q Kc
then the reaction must go in reverse to achieve
equilibrium if Q=Kc the reaction is at
equilibrium
...
... same formula as Kc but means that the reaction is not necessarily at equilibrium. (Instantaneous) • If Q
An Overview of Organic Reactions
... The energy is mostly determined by the type of bond, independent of the molecule § The C-H bond in methane requires a net energy input of 106 kcal/mol to be broken at 25 ºC. § Table 6.3 lists energies for many bond types Changes in bonds can be used to calculate net changes in heat ...
... The energy is mostly determined by the type of bond, independent of the molecule § The C-H bond in methane requires a net energy input of 106 kcal/mol to be broken at 25 ºC. § Table 6.3 lists energies for many bond types Changes in bonds can be used to calculate net changes in heat ...
Free Energy I
... Irreversible Processes An irreversible process is one in which the system and surroundings cannot be restored to their original state by exactly reversing the change. dropping a vase and breaking it reacting hydrogen and oxygen to form water burning a match ...
... Irreversible Processes An irreversible process is one in which the system and surroundings cannot be restored to their original state by exactly reversing the change. dropping a vase and breaking it reacting hydrogen and oxygen to form water burning a match ...
STUDY GUIDE
... is neither created nor destroyed. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. The general types of chemical reactions are combination, decomposition, single-replacement, doublereplacement, and combustion reactions. Net ionic equations show only th ...
... is neither created nor destroyed. In order to show that mass is conserved during a reaction, a chemical equation must be balanced. The general types of chemical reactions are combination, decomposition, single-replacement, doublereplacement, and combustion reactions. Net ionic equations show only th ...