Chemistry - StudyTime NZ
... Eventually, every reac>on will reach a point where all of the available molecules have performed collisions with each other. At this point, all of the reactants have been used up and hence no mor ...
... Eventually, every reac>on will reach a point where all of the available molecules have performed collisions with each other. At this point, all of the reactants have been used up and hence no mor ...
File
... 68. The phase diagram for a pure substance is shown above. The solid and gaseous phases of the substances can exist in equilibrium at conditions corresponding to which of the following? A) Point I only B) Point III only C) Any point on the curve from I to II. D) Any point on the curve from II to II ...
... 68. The phase diagram for a pure substance is shown above. The solid and gaseous phases of the substances can exist in equilibrium at conditions corresponding to which of the following? A) Point I only B) Point III only C) Any point on the curve from I to II. D) Any point on the curve from II to II ...
Gas-forming Reactions
... In addition to precipitation and neutralization reactions, aqueous ions can participate in oxidation-reduction reactions. Oxidation-reduction reactions involve the transfer of electrons from one chemical species to another. When magnesium burns, it combines with oxygen to form magnesium oxide. This ...
... In addition to precipitation and neutralization reactions, aqueous ions can participate in oxidation-reduction reactions. Oxidation-reduction reactions involve the transfer of electrons from one chemical species to another. When magnesium burns, it combines with oxygen to form magnesium oxide. This ...
Thermochemistry
... and chemical changes occur because that is the path of least resistance. The production and usage of energy that occurs in reactions have enormous impacts on society. People are making big bucks as they try to find new ways to harness energy (think of nuclear energy, solar energy, and fossil fuels). ...
... and chemical changes occur because that is the path of least resistance. The production and usage of energy that occurs in reactions have enormous impacts on society. People are making big bucks as they try to find new ways to harness energy (think of nuclear energy, solar energy, and fossil fuels). ...
Gas and Thermo Notes
... following conversion factor to change the units into joules: 101.3 J = 1 L•atm 1. Calculate ∆E for a system undergoing an endothermic process in which 15.6 kJ of heat flows and where 1.4 kJ of work is done on the system. ...
... following conversion factor to change the units into joules: 101.3 J = 1 L•atm 1. Calculate ∆E for a system undergoing an endothermic process in which 15.6 kJ of heat flows and where 1.4 kJ of work is done on the system. ...
Name:__Grading key
... Welcome to the first hourly exam for Chemistry 125/126. This exam consists of 7 questions worth a total of 75 points plus a bonus question worth 3 points for a possible total of 78 points. It is 10 pages long; 8 pages of questions including a bonus question and periodic tables (page 10). To receive ...
... Welcome to the first hourly exam for Chemistry 125/126. This exam consists of 7 questions worth a total of 75 points plus a bonus question worth 3 points for a possible total of 78 points. It is 10 pages long; 8 pages of questions including a bonus question and periodic tables (page 10). To receive ...
5.7 Quantity Relationships in Chemical Reactions
... reagent runs out before the other. The significance of this is that the reagent that runs out first defines or limits the amount of product made. We call the reagent that runs out first the limiting reagent. The one that does not run out is called the excess reagent. If the reagents are mixed in non ...
... reagent runs out before the other. The significance of this is that the reagent that runs out first defines or limits the amount of product made. We call the reagent that runs out first the limiting reagent. The one that does not run out is called the excess reagent. If the reagents are mixed in non ...
CHE 1401 - Summer 2012 - Chapter 5 Homework 5 (Chapter 5
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
... B) A negative ΔH corresponds to an exothermic process. C) ΔE = Efinal - Einitial D) Energy lost by the system must be gained by the surroundings. E) 1 cal = 4.184 J (exactly) 9) The British thermal unit (Btu) is commonly used in engineering applications. A Btu is defined as the amount of heat requir ...
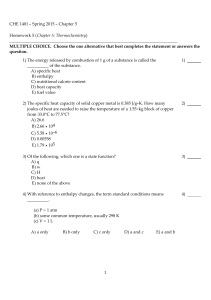
CHE 1401 - Spring 2015 - Chapter 5 Homework 5 (Chapter 5
... B) The system gains heat and has work done on it by the surroundings. C) The system loses heat and does work on the surroundings. D) The system loses heat and has work done on it by the surroundings. E) None of the above is correct. ...
... B) The system gains heat and has work done on it by the surroundings. C) The system loses heat and does work on the surroundings. D) The system loses heat and has work done on it by the surroundings. E) None of the above is correct. ...
CHE 1401 - Fall 2016 - Chapter 5 Homework 5 (Chapter 5
... Ti (s) + O2 (g) → TiO2 (s) When 0.721 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00°C to 53.80°C. In a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kJ/K. The heat of reaction for the combustion of a mole ...
... Ti (s) + O2 (g) → TiO2 (s) When 0.721 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00°C to 53.80°C. In a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kJ/K. The heat of reaction for the combustion of a mole ...
Chemistry
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
... 11. use chemical skills in contexts which bring together different areas of the subject. These assessment objectives cannot be precisely specified in the Syllabus Content because questions testing such skills may be based on information which is unfamiliar to the candidate. In answering such questio ...
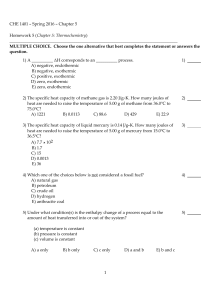
CHE 1401 - Spring 2016 - Chapter 5 Homework 5 (Chapter 5
... the heat capacity of the calorimeter is measured to be 9.84 kJ/K. The heat of reaction for the combustion of a mole of Ti in this calorimeter is __________ kJ/mol. A) -0.154 B) -1.49 × 104 C) 2.67 D) -311 E) 4.98 31) The British thermal unit (Btu) is commonly used in engineering applications. A Btu ...
... the heat capacity of the calorimeter is measured to be 9.84 kJ/K. The heat of reaction for the combustion of a mole of Ti in this calorimeter is __________ kJ/mol. A) -0.154 B) -1.49 × 104 C) 2.67 D) -311 E) 4.98 31) The British thermal unit (Btu) is commonly used in engineering applications. A Btu ...
Hein and Arena - faculty at Chemeketa
... In 1888, the French chemist Henri LeChatelier This generalization, known as set forth a far-reaching generalization on the LeChatelier’s Principle, states behavior of equilibrium systems. If a stress or strain is applied to a system in equilibrium, the system will respond in such a way as to reliev ...
... In 1888, the French chemist Henri LeChatelier This generalization, known as set forth a far-reaching generalization on the LeChatelier’s Principle, states behavior of equilibrium systems. If a stress or strain is applied to a system in equilibrium, the system will respond in such a way as to reliev ...
Topic 6 Kinetics File
... On the grids below, plot energy diagrams for 1–3 below, given the following information, and answer the questions. For number 4, study the energy diagram and answer the questions. 1. On the grid to the right, plot the energy diagram given the following information. Potential Energy of reactants: 250 ...
... On the grids below, plot energy diagrams for 1–3 below, given the following information, and answer the questions. For number 4, study the energy diagram and answer the questions. 1. On the grid to the right, plot the energy diagram given the following information. Potential Energy of reactants: 250 ...
Chemistry 12 – Unit 3 – Chapter 5 – Thermochemistry
... ∆H0reaction = Σ n∆H0f products - Σ n∆H0f reactants ∆H0rxn = [6 mol x ∆H0f of H2O(l) + 6 mol x ∆H0f of CO2(g)] – [2 mol x ∆H0f of C3H6(g) + 9 mol x ∆H0f of O2(g)] ∆H0rxn = [ 6 mol x(-285.8 kJ/mol) + 6 mol x (-393.5 kJ/mol) ] – [ 2 mol x (17.8 kJ/mol) + 9 mol x (0 kJ/mol)] ∆H0rxn = [ -1714.8 kJ + -236 ...
... ∆H0reaction = Σ n∆H0f products - Σ n∆H0f reactants ∆H0rxn = [6 mol x ∆H0f of H2O(l) + 6 mol x ∆H0f of CO2(g)] – [2 mol x ∆H0f of C3H6(g) + 9 mol x ∆H0f of O2(g)] ∆H0rxn = [ 6 mol x(-285.8 kJ/mol) + 6 mol x (-393.5 kJ/mol) ] – [ 2 mol x (17.8 kJ/mol) + 9 mol x (0 kJ/mol)] ∆H0rxn = [ -1714.8 kJ + -236 ...
Redox Reactions - hrsbstaff.ednet.ns.ca
... Oxidation and reduction reaction = redox rxn Oxidation is loss of electrons and reduction is gain of electrons = transfer of electrons Those 2 reactions are occurring simultaneously ...
... Oxidation and reduction reaction = redox rxn Oxidation is loss of electrons and reduction is gain of electrons = transfer of electrons Those 2 reactions are occurring simultaneously ...