Chemistry I
... Isotopes are variants of atoms of a particular chemical element, differing in the numbers of neutrons and in the atomic mass. They contain the same number of protons, but a different number of neutrons. Examples: Examples: ...
... Isotopes are variants of atoms of a particular chemical element, differing in the numbers of neutrons and in the atomic mass. They contain the same number of protons, but a different number of neutrons. Examples: Examples: ...
Chemistry - Textbooks Online
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
Elements, Compounds, and Mixtures
... A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
... A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
as a PDF
... in which all substances are tri-f and there is no change in the 4f electron populations. With three bonding electrons per metal atom, there are six such electrons on each side of Eq. (11). Three can be allocated to the formation of bonds with chlorine, and three to the formation of multi-centred Gd– ...
... in which all substances are tri-f and there is no change in the 4f electron populations. With three bonding electrons per metal atom, there are six such electrons on each side of Eq. (11). Three can be allocated to the formation of bonds with chlorine, and three to the formation of multi-centred Gd– ...
Physical Earth Daily Learning Guide DRAFT - Burlington
... bonds. Such compounds consist of welldefined molecules. Formulas of covalent compounds represent the types and number of atoms of each element in each molecule. ...
... bonds. Such compounds consist of welldefined molecules. Formulas of covalent compounds represent the types and number of atoms of each element in each molecule. ...
Biomolecular modeling
... • interactions mediated by, and resulting directly from the presence of a covalent bond between the atoms. We usually put springs between the atoms and have to care about bond and dihedral angles. With this treatment, we describe all the quantummechanical phenomena like exchange and correlation usin ...
... • interactions mediated by, and resulting directly from the presence of a covalent bond between the atoms. We usually put springs between the atoms and have to care about bond and dihedral angles. With this treatment, we describe all the quantummechanical phenomena like exchange and correlation usin ...
The Atomic Theory Chem 111
... ions (cations) are considered ionic. In chemical reactions the lose or gain of electrons by an atom or molecule results in a charged particle called an ion. Ion that contain more than one atom is a polyatomic ion. Example: NH4+ or NO3-. An ion with only on atom is a monatomic ion. Example: Fe3+ or S ...
... ions (cations) are considered ionic. In chemical reactions the lose or gain of electrons by an atom or molecule results in a charged particle called an ion. Ion that contain more than one atom is a polyatomic ion. Example: NH4+ or NO3-. An ion with only on atom is a monatomic ion. Example: Fe3+ or S ...
chem 13 news 2010 - University of Waterloo
... 17 Exactly 850 mL of O2 gas is collected over water at 30.0 oC using the setup below. Given that the barometric pressure was 98.5 kPa and the vapour pressure of water is 4.24 kPa at 30 oC, what volume would the pure O2 gas occupy at 98.5 kPa and 30 oC? ...
... 17 Exactly 850 mL of O2 gas is collected over water at 30.0 oC using the setup below. Given that the barometric pressure was 98.5 kPa and the vapour pressure of water is 4.24 kPa at 30 oC, what volume would the pure O2 gas occupy at 98.5 kPa and 30 oC? ...
PPT - Unit 5
... -(C2H2(g) + 5/2O2(g) → 2CO2(g) + H2O(l) ΔH = -1300. kJ) 2( C(s) + O2(g) → CO2(g) ) 2(ΔH = -394 kJ) H2(g) + 1/2O2(g) → H2O(l) ΔH = -286 kJ Calculate ΔH for the following reaction: 2C(s) + H2(g) → C2H2(g) 2C(s) + 2O2(g) → 2CO2(g) ΔH = -788 kJ 2CO2(g) + H2O(l) → C2H2(g) + 5/2O2(g) ΔH = +1300 kJ H2(g) + ...
... -(C2H2(g) + 5/2O2(g) → 2CO2(g) + H2O(l) ΔH = -1300. kJ) 2( C(s) + O2(g) → CO2(g) ) 2(ΔH = -394 kJ) H2(g) + 1/2O2(g) → H2O(l) ΔH = -286 kJ Calculate ΔH for the following reaction: 2C(s) + H2(g) → C2H2(g) 2C(s) + 2O2(g) → 2CO2(g) ΔH = -788 kJ 2CO2(g) + H2O(l) → C2H2(g) + 5/2O2(g) ΔH = +1300 kJ H2(g) + ...
Tutorial – Mass mole conversions Std 3e
... With this step, we went from grams to MOLES on our diagram. But we need the moles of O2 not H2 . This is the step that requires the extra MOLES I put into the diagram above. Now it’s time to cancel the units we can and also change moles of H2 to moles of O2 . For that we need to use the mole ratio f ...
... With this step, we went from grams to MOLES on our diagram. But we need the moles of O2 not H2 . This is the step that requires the extra MOLES I put into the diagram above. Now it’s time to cancel the units we can and also change moles of H2 to moles of O2 . For that we need to use the mole ratio f ...
IB:Enthalpy Review Questions
... a. Calculate the value of ∆H0 for the above reaction and state what information the sign of ∆H0 provides about this reaction. b. Calculate ∆S0 for the reaction and state the meaning of the sign of ∆S0 obtained. c. Identify a thermodynamic function that can be used to predict reaction spontaneity, at ...
... a. Calculate the value of ∆H0 for the above reaction and state what information the sign of ∆H0 provides about this reaction. b. Calculate ∆S0 for the reaction and state the meaning of the sign of ∆S0 obtained. c. Identify a thermodynamic function that can be used to predict reaction spontaneity, at ...
Chapter 2
... Predicting Formulas Let’s consider a compound containing Mg and N. • The common charge on Mg is +2 (or Mg2+). • The common charge on N is –3 (or N3-). • Since we want to make a neutral (uncharged) compound, the total charges from the cations and anions must cancel-out (or sum to zero). • Therefore, ...
... Predicting Formulas Let’s consider a compound containing Mg and N. • The common charge on Mg is +2 (or Mg2+). • The common charge on N is –3 (or N3-). • Since we want to make a neutral (uncharged) compound, the total charges from the cations and anions must cancel-out (or sum to zero). • Therefore, ...
avogadro exam 2001 - University of Waterloo
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send you a report on your performance. Top performers are eligible for a prize. ...
... This exam is being written by several thousand students. Please be sure that you follow the instructions below. We'll send you a report on your performance. Top performers are eligible for a prize. ...
Gmelin Tips and Reminders
... • Linearized Structure Formula: LSF: a linear representation of a compound’s structure. This can include isotopes. Compounds can consist of more than one fragment being separated in the linearized structure formula field by an asterisk (*); formulas within systems are separated by a hush mark (#); d ...
... • Linearized Structure Formula: LSF: a linear representation of a compound’s structure. This can include isotopes. Compounds can consist of more than one fragment being separated in the linearized structure formula field by an asterisk (*); formulas within systems are separated by a hush mark (#); d ...
CHEM1901/3 Tutorials The problem sheets on the following pages
... century. Each chemical element was assigned an atomic mass that was assumed not to change as its atoms underwent chemical change. Any heat, work, or other energy produced by a reaction was said to have been derived from chemical energy. Mass did not enter into the discussion of energy. Chemists must ...
... century. Each chemical element was assigned an atomic mass that was assumed not to change as its atoms underwent chemical change. Any heat, work, or other energy produced by a reaction was said to have been derived from chemical energy. Mass did not enter into the discussion of energy. Chemists must ...
avogadro exam 2012 - University of Waterloo
... • Mark only one answer for each question. • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make t ...
... • Mark only one answer for each question. • Questions are all of the same value. • There is a penalty (1/4 off) for each incorrect answer, but no penalty if you do not answer. 7. Take care that you make firm, black pencil marks, just filling the oval. Be careful that any erasures are complete—make t ...
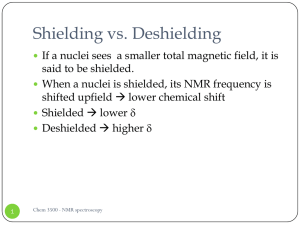
Shielding vs. Deshielding
... When a chemical bond is placed in a external magnetic field, it will generate a local field ...
... When a chemical bond is placed in a external magnetic field, it will generate a local field ...
AP Chemistry: Bonding Multiple Choice
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
... (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg. (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium. (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere. (E) lowest temperature above which a substance c ...
Chemistry(I) Final Exam 1/11/2008
... Consider the properties of H2(g) and H2O(g). (a) Assume they are ideal gases. The root mean spead of H2O(g) is 640 m/s at 25°C. Calculate the root mean spead of H2(g)? (b) The van der Waals a parameters of these two gases are 5.536 and 0.2476 L2·atm/mol2. Use your knowledge of the factors governing ...
... Consider the properties of H2(g) and H2O(g). (a) Assume they are ideal gases. The root mean spead of H2O(g) is 640 m/s at 25°C. Calculate the root mean spead of H2(g)? (b) The van der Waals a parameters of these two gases are 5.536 and 0.2476 L2·atm/mol2. Use your knowledge of the factors governing ...
Chapter 2 Atoms, Molecules, and Ions
... • Molecular formulas give the exact number of atoms of each element in a compound. Example: ethane: Empirical formula: CH3 Molecular formula: C2H6 ...
... • Molecular formulas give the exact number of atoms of each element in a compound. Example: ethane: Empirical formula: CH3 Molecular formula: C2H6 ...
Glossary - Chemistry (Intro)
... Nuclear C.: The study of the structure of nuclei, of the changes this structure undergoes, and of the consequences of those changes for chemistry. Thermo C.: The study of heat changes in chemical reactions. Closed System: A system that allows the exchange of energy (usually in the form of heat) but ...
... Nuclear C.: The study of the structure of nuclei, of the changes this structure undergoes, and of the consequences of those changes for chemistry. Thermo C.: The study of heat changes in chemical reactions. Closed System: A system that allows the exchange of energy (usually in the form of heat) but ...
Proton Resonance Frequencies in Several Organophosphorus Acids
... cannot be elminated from consideration. However, existence of an extended polymer seems less likely since preservation of an structure of this type in a polar medium capable of H-bonding would require that the Hbonds be unusually strong. A close analogy is the observation that in a number of polar s ...
... cannot be elminated from consideration. However, existence of an extended polymer seems less likely since preservation of an structure of this type in a polar medium capable of H-bonding would require that the Hbonds be unusually strong. A close analogy is the observation that in a number of polar s ...
Textbook Answer Keys - Mr. Massey`s Chemistry Pages
... Opinions or conclusions should be presented clearly and supported by appropriate evidence. [Assessment Objective 3] The Dalton model of the atom as an indivisible particles was changed as result of Thomson’s experiment; without the use of high voltage cathode rays and magnetism, Thomson would not ha ...
... Opinions or conclusions should be presented clearly and supported by appropriate evidence. [Assessment Objective 3] The Dalton model of the atom as an indivisible particles was changed as result of Thomson’s experiment; without the use of high voltage cathode rays and magnetism, Thomson would not ha ...
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms).Each contributing structure can be represented by a Lewis structure, with only an integer number of covalent bonds between each pair of atoms within the structure. Several Lewis structures are used collectively to describe the actual molecular structure, which is an approximate intermediate between the canonical forms called a resonance hybrid. Contributing structures differ only in the position of electrons, not in the position of nuclei.Electron delocalization lowers the potential energy of the substance and thus makes it more stable than any of the contributing structures. The difference between the potential energy of the actual structure and that of the contributing structure with the lowest potential energy is called the resonance energy or delocalization energy.Resonance is distinguished from tautomerism and conformational isomerism, which involve the formation of isomers, thus the rearrangement of the nuclear positions.