File
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
... valence shell) usually determine how an atom will react Atoms are stable when their outer energy level is full Atoms can gain or lose electrons to become stable ...
A Plausible Explanation of the double-slit Experiment in
... energy to cause it to 'pop' (much like popcorn at seemingly random manner once a seed has absorbed enough heat energy). The parts of the detection screen that over time are illuminated more frequently by energy will of course show more 'popping'. The emission of an electron at the source is a separa ...
... energy to cause it to 'pop' (much like popcorn at seemingly random manner once a seed has absorbed enough heat energy). The parts of the detection screen that over time are illuminated more frequently by energy will of course show more 'popping'. The emission of an electron at the source is a separa ...
Advanced electronic bonding and how these affect molecular shapes
... • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energy levels are named 1, 2, 3, 4, 5, 6, 7, 8 and so on. (So far the heaviest element discovered ever has o ...
... • These energy levels are called shells. • Electrons jump to higher energy levels when provided with energy, but will automatically drop back down to the lowest energy level possible. • These energy levels are named 1, 2, 3, 4, 5, 6, 7, 8 and so on. (So far the heaviest element discovered ever has o ...
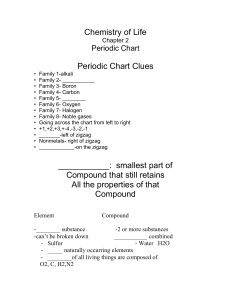
Chemistry of Life
... • Oxygen and Hydrogen are bonded together by ________ electrons, but the Oxygen atom _________the shared electrons closer to it, creating negative and positive sides of the water molecules. Water has a partial negative charge due to the extra unshared e- that Oxygen and a partial + charge near the h ...
... • Oxygen and Hydrogen are bonded together by ________ electrons, but the Oxygen atom _________the shared electrons closer to it, creating negative and positive sides of the water molecules. Water has a partial negative charge due to the extra unshared e- that Oxygen and a partial + charge near the h ...
Lecture1
... Absorption of photon by atomic electron Ejection of electron from atom Energy of outgoing electron ...
... Absorption of photon by atomic electron Ejection of electron from atom Energy of outgoing electron ...
SEMESTER 1 EXAM Prblms/Short Ans
... 7. Illustration: In the boxes provided, draw and label a picture of the atomic model based on J.J. Thomson’s experiment, Ernest Rutherford’s experiment, Niels Bohr’s experiment and the Quantum model of the atom. Show protons, neutrons and electrons and their believed relationship to each other with ...
... 7. Illustration: In the boxes provided, draw and label a picture of the atomic model based on J.J. Thomson’s experiment, Ernest Rutherford’s experiment, Niels Bohr’s experiment and the Quantum model of the atom. Show protons, neutrons and electrons and their believed relationship to each other with ...
Nordheim, L. “Electron emission in intense electric fields,”
... At intermediate strengths it does not appear to have been tested quantitatively and therefore yet awaits experimental and theoretical investigation. On one other deduction, however, they lay great emphasis, and this we find liable to mislead. They assert that a distinction should be drawn between th ...
... At intermediate strengths it does not appear to have been tested quantitatively and therefore yet awaits experimental and theoretical investigation. On one other deduction, however, they lay great emphasis, and this we find liable to mislead. They assert that a distinction should be drawn between th ...
Class 22
... Only way for individual atoms to give off energy is as light. Atoms are lazy - always want to go back to lowest energy state. 2. Excited atom ..electron 3. Electron 1. Fast electron in atom goes to higher jumps back to hits atom energy low energy ...
... Only way for individual atoms to give off energy is as light. Atoms are lazy - always want to go back to lowest energy state. 2. Excited atom ..electron 3. Electron 1. Fast electron in atom goes to higher jumps back to hits atom energy low energy ...
Chapter 28
... Difficulties with the Rutherford Model • Atoms emit certain discrete characteristic frequencies of electromagnetic radiation but the Rutherford model is unable to explain this phenomena • Rutherford’s electrons are undergoing a centripetal acceleration and so should radiate electromagnetic waves of ...
... Difficulties with the Rutherford Model • Atoms emit certain discrete characteristic frequencies of electromagnetic radiation but the Rutherford model is unable to explain this phenomena • Rutherford’s electrons are undergoing a centripetal acceleration and so should radiate electromagnetic waves of ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.