Mathcad - ROOTS.mcd
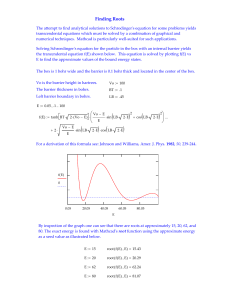
... the trancendental equation f(E) shown below. This equation is solved by plotting f(E) vs E to find the approximate values of the bound energy states. The box is 1 bohr wide and the barrier is 0.1 bohr thick and located in the center of the box. Vo is the barrier height in hartrees. ...
... the trancendental equation f(E) shown below. This equation is solved by plotting f(E) vs E to find the approximate values of the bound energy states. The box is 1 bohr wide and the barrier is 0.1 bohr thick and located in the center of the box. Vo is the barrier height in hartrees. ...
2011 Chem Facts Key
... 18. Electrons can be excited to jump to higher energy levels. They emit energy as light when they fall from higher energy levels back down to lower (ground state) energy levels. Bright line spectra are produced. 19. Elements are pure substances composed of atoms with the same atomic number. They can ...
... 18. Electrons can be excited to jump to higher energy levels. They emit energy as light when they fall from higher energy levels back down to lower (ground state) energy levels. Bright line spectra are produced. 19. Elements are pure substances composed of atoms with the same atomic number. They can ...
lecture31
... Because of that, we can expect 3 independent external quantum numbers. However, the potential energy is function of one coordinate, r. Because of that, electron’s energy depends only on one of these 3 numbers. In addition, an electron has one internal quantum number. ...
... Because of that, we can expect 3 independent external quantum numbers. However, the potential energy is function of one coordinate, r. Because of that, electron’s energy depends only on one of these 3 numbers. In addition, an electron has one internal quantum number. ...
chemia simr01 en - Leszek Niedzicki
... accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared) is ‘closer’ to the other atom; • Hydrogen is ‘looking’ f ...
... accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared) is ‘closer’ to the other atom; • Hydrogen is ‘looking’ f ...
Chapter 30: Quantum Physics
... energy of the electron by the kinetic energy of the proton to calculate their ratio. Solution: 1. (a) The proton and electron have the same momentum because they have the same de Broglie wavelength. The kinetic energy, K p 2 2m , is inversely proportional to the mass, and me mp . For identical m ...
... energy of the electron by the kinetic energy of the proton to calculate their ratio. Solution: 1. (a) The proton and electron have the same momentum because they have the same de Broglie wavelength. The kinetic energy, K p 2 2m , is inversely proportional to the mass, and me mp . For identical m ...
lecture31
... •Because of that, we can expect 3 independent external quantum numbers. •However, the potential energy is function of one coordinate, r. •Because of that, electron’s energy depends only on one of these 3 numbers. •In addition, an electron has one internal quantum number. ...
... •Because of that, we can expect 3 independent external quantum numbers. •However, the potential energy is function of one coordinate, r. •Because of that, electron’s energy depends only on one of these 3 numbers. •In addition, an electron has one internal quantum number. ...
Chapter 3
... levels, an electron can have. For each energy level, the Schordinger’s equation also leads to a mathematical expression called an atomic orbital which describes the probability of finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a regio ...
... levels, an electron can have. For each energy level, the Schordinger’s equation also leads to a mathematical expression called an atomic orbital which describes the probability of finding an electron at various locations around the nucleus of. An atomic orbitals is represented pictorially as a regio ...
AP Chemistry Summer Study Guide
... Attached you will find summer work which will review topics that were learned in Honors Chemistry. It is imperative that you review during the summer. We will begin by applying the concepts you have already mastered this past year and begin looking at problems in a new and exciting way. You may use ...
... Attached you will find summer work which will review topics that were learned in Honors Chemistry. It is imperative that you review during the summer. We will begin by applying the concepts you have already mastered this past year and begin looking at problems in a new and exciting way. You may use ...
slicing and dicing photons - Department of Physics and Astronomy
... complications associated with the indirect bandgap in the electronic structure of silicon. However, in the characterization of cooling dynamics for several other types of NCs such as InP, CdSe and PbSe, the cooling times fall generally in the subpicosecond range. It is noted by Timmerman et al. that ...
... complications associated with the indirect bandgap in the electronic structure of silicon. However, in the characterization of cooling dynamics for several other types of NCs such as InP, CdSe and PbSe, the cooling times fall generally in the subpicosecond range. It is noted by Timmerman et al. that ...
Final
... (4) Consider the Bogoliubov de Gennes equations for an interface between a normal metal and a superconductor with gap ∆. Solve these equations for the effectively 1-dim case where an electron is incident on the interface from the normal side with sufficiently high energy to induce injection of a Bog ...
... (4) Consider the Bogoliubov de Gennes equations for an interface between a normal metal and a superconductor with gap ∆. Solve these equations for the effectively 1-dim case where an electron is incident on the interface from the normal side with sufficiently high energy to induce injection of a Bog ...
Flame Test Lab
... energy of each photon is described by the equation E = hv, where h is Planck’s constant (6.63 x 10 -34 Js) and v is the frequency of the radiation. If the wavelength of the released photon is between 400 nm and 700 nm, the energy is emitted as visible light. The color of the light depends on the spe ...
... energy of each photon is described by the equation E = hv, where h is Planck’s constant (6.63 x 10 -34 Js) and v is the frequency of the radiation. If the wavelength of the released photon is between 400 nm and 700 nm, the energy is emitted as visible light. The color of the light depends on the spe ...
Exercises - Galena Park ISD
... 24. The electron microscope makes use of the electrons. 25. Explain why an electron microscope can distinguish much more detail than an optical microscope. The much shorter wavelength of the electrons allows details to be distinguished that are thousands of times smaller than is possible with optica ...
... 24. The electron microscope makes use of the electrons. 25. Explain why an electron microscope can distinguish much more detail than an optical microscope. The much shorter wavelength of the electrons allows details to be distinguished that are thousands of times smaller than is possible with optica ...
Electron Configuration
... set of four quantum numbers. This means no atomic orbital can contain more than TWO electrons and the electrons must be of opposite spin if they are to form a pair within an orbital. ...
... set of four quantum numbers. This means no atomic orbital can contain more than TWO electrons and the electrons must be of opposite spin if they are to form a pair within an orbital. ...
Energy Transformation
... and the interior of the Earth, all have "nuclear reactions" as the source of their energy, that is, reactions that involve changes in the structure of the nuclei of atoms. In the Sun, hydrogen nuclei fuse (combine) together to make helium nuclei, in a process called fusion, which releases energy. In ...
... and the interior of the Earth, all have "nuclear reactions" as the source of their energy, that is, reactions that involve changes in the structure of the nuclei of atoms. In the Sun, hydrogen nuclei fuse (combine) together to make helium nuclei, in a process called fusion, which releases energy. In ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.























