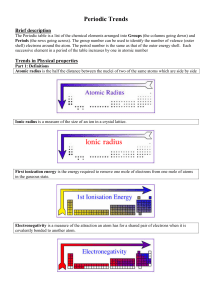
CHM_101_ASSIGNMENT_COPY_1_2
... solid NaOH to a buffer solution that consists of 0.15M sodium acetate and 0.15M acetic acid solution, if we assume that there is no change in volume (Ka = 1.8 x 10-5). 2. (a) The rate constant of a first order reaction is 2.5 ×10 -6/s and the initial concentration is 0.1moldm-3, what is the initial ...
... solid NaOH to a buffer solution that consists of 0.15M sodium acetate and 0.15M acetic acid solution, if we assume that there is no change in volume (Ka = 1.8 x 10-5). 2. (a) The rate constant of a first order reaction is 2.5 ×10 -6/s and the initial concentration is 0.1moldm-3, what is the initial ...
Name: Chapter 3 Reading Guide: Molecules, Compounds, and
... 3.6 Molecular Compounds: Formulas and Names (p. 101-105) The formula for a molecular compound ______________________ readily be determined from its constituent elements because the same combination of elements may form many ____________________ molecular compounds, each with a _____________________ ...
... 3.6 Molecular Compounds: Formulas and Names (p. 101-105) The formula for a molecular compound ______________________ readily be determined from its constituent elements because the same combination of elements may form many ____________________ molecular compounds, each with a _____________________ ...
Modern Physics 3-Atomic Physics
... a chain of carbon atoms. Electrons of the bonds along the chain of carbon atoms are shared among the atoms in the chain, but are repelled by the nitrogen-containing rings at the end of the chain. These electrons are thus free to move along the chain but not beyond its ends. They look very much like ...
... a chain of carbon atoms. Electrons of the bonds along the chain of carbon atoms are shared among the atoms in the chain, but are repelled by the nitrogen-containing rings at the end of the chain. These electrons are thus free to move along the chain but not beyond its ends. They look very much like ...
Chapter 4 Notes - Atomic Theory
... A skeleton equation shows only the formulas of the elements/compounds Shows atoms, but is not balanced K(s) + O2 (g) K2O(s) A balanced chemical equation shows the correct number of each atom Balancing ensures that the number of each atom is the same on both sides of the reaction arrow ...
... A skeleton equation shows only the formulas of the elements/compounds Shows atoms, but is not balanced K(s) + O2 (g) K2O(s) A balanced chemical equation shows the correct number of each atom Balancing ensures that the number of each atom is the same on both sides of the reaction arrow ...
Text S1.
... phase consisted of two stages: In the first stage, the conformers of each dipeptide were combined. In the second stage, the atomic charges of equivalent atoms were equated and the charges of terminal groups and heavy atoms were fixed. All fitting calculations were done with the RESP module of Amber1 ...
... phase consisted of two stages: In the first stage, the conformers of each dipeptide were combined. In the second stage, the atomic charges of equivalent atoms were equated and the charges of terminal groups and heavy atoms were fixed. All fitting calculations were done with the RESP module of Amber1 ...
High School Curriculum Standards: Chemistry
... 3.1u Elements are substances that are composed of atoms that have the same atomic number. Elements cannot be broken down by chemical change. 3.1v Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases ...
... 3.1u Elements are substances that are composed of atoms that have the same atomic number. Elements cannot be broken down by chemical change. 3.1v Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases ...
Ionic Bonding
... eight electrons in their outer energy levels (or two in the case of helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impression that when other atoms react, they try to organize things such th ...
... eight electrons in their outer energy levels (or two in the case of helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impression that when other atoms react, they try to organize things such th ...
Electronic Structure of Atoms
... •Line spectra of many electron atoms show each line as a closely spaced pair of lines. •Stern and Gerlach designed an experiment to determine why. •A beam of atoms was passed through a slit and into a magnetic field and the atoms were then detected. •Two spots were found: one with the electrons spin ...
... •Line spectra of many electron atoms show each line as a closely spaced pair of lines. •Stern and Gerlach designed an experiment to determine why. •A beam of atoms was passed through a slit and into a magnetic field and the atoms were then detected. •Two spots were found: one with the electrons spin ...
Effects of antioxidants for the degradation of flame
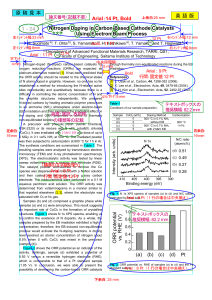
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
Lecture notes lecture 13 (quantum physics)
... the atom is much more complex than the traditional model. The world of subatomic particles is a very bizarre one, filled with quantum probabilities and organized chaos. For example, the exact position and velocity of an electron is very hard to find because attempts to "see" it involve bouncing othe ...
... the atom is much more complex than the traditional model. The world of subatomic particles is a very bizarre one, filled with quantum probabilities and organized chaos. For example, the exact position and velocity of an electron is very hard to find because attempts to "see" it involve bouncing othe ...
Study Guide for Exam 2_Sp12
... What is the difference between molecular mass and formula mass? You should be able to use the periodic table and a chemical formula to calculate the molecular mass or the formula mass of any compound or element. You need to memorize Avogadro’s number. What is a mole? You should be able to use Avogad ...
... What is the difference between molecular mass and formula mass? You should be able to use the periodic table and a chemical formula to calculate the molecular mass or the formula mass of any compound or element. You need to memorize Avogadro’s number. What is a mole? You should be able to use Avogad ...
> >
... You will get to use the AP exam equation sheets on the test, but that’s it. So if think you’ll need any additional equations, memorize them. And don’t forget those solubility rules. Chapter 1 – Matter, Measure ...
... You will get to use the AP exam equation sheets on the test, but that’s it. So if think you’ll need any additional equations, memorize them. And don’t forget those solubility rules. Chapter 1 – Matter, Measure ...
Electron Configuration Notes File
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
... Steps to Writing Electron Configuration 1. Determine the # of electrons 2. Use the redesigned PT to get the configuration 3. Superscripts will equal the electrons ...
lecture 10
... • He, Ne, Ar: rare gases … adding electron to filled shell … must increase n • Be, Mg: alkali earths … adding electron to filled sub-shell … must increase l • N: Hund’s rule stability… adding electron to ½-filled degenerate p-shell ...
... • He, Ne, Ar: rare gases … adding electron to filled shell … must increase n • Be, Mg: alkali earths … adding electron to filled sub-shell … must increase l • N: Hund’s rule stability… adding electron to ½-filled degenerate p-shell ...
2. Characterisation techniques
... or beams of electrons or neutrons with similar wavelength. So, through X-ray spectra one can identify and analyse any crystalline matter. The degree of crystallinity or order will conditionate the quality of the obtained result. In order to do this, a diffractometer is needed. Basically, an X-ray di ...
... or beams of electrons or neutrons with similar wavelength. So, through X-ray spectra one can identify and analyse any crystalline matter. The degree of crystallinity or order will conditionate the quality of the obtained result. In order to do this, a diffractometer is needed. Basically, an X-ray di ...
Lectures 1-2: Introduction to Atomic Spectroscopy Types of Spectra
... o Continuous spectrum: Produced by solids, liquids & dense gases produce - no “gaps” in wavelength of light produced: ...
... o Continuous spectrum: Produced by solids, liquids & dense gases produce - no “gaps” in wavelength of light produced: ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.