02_Lecture - WordPress.com
... Law of Multiple Proportions If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the ...
... Law of Multiple Proportions If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the ...
Chemistry Revision Checklist F4 2017 (inc F3)
... Name and draw the structures of the unbranched alkanes, alkenes (not cis-trans), alcohols and acids containing up to four carbon atoms per molecule State the type of compound present, given a chemical name ending in -ane, -ene, -ol, or -oic acid or a molecular structure Describe the concept of homol ...
... Name and draw the structures of the unbranched alkanes, alkenes (not cis-trans), alcohols and acids containing up to four carbon atoms per molecule State the type of compound present, given a chemical name ending in -ane, -ene, -ol, or -oic acid or a molecular structure Describe the concept of homol ...
Endothermic reactions
... You have seen many reactions that release energy. Chemical reactions that release energy are called exergonic (ek sur GAH nihk) reactions. In these reactions, less energy is required to break the original bonds than is released when new bonds are formed. As a result, some form of energy, such as lig ...
... You have seen many reactions that release energy. Chemical reactions that release energy are called exergonic (ek sur GAH nihk) reactions. In these reactions, less energy is required to break the original bonds than is released when new bonds are formed. As a result, some form of energy, such as lig ...
Building the sense of math in physics activities
... net flow from the more dense concentration side to the less. B. The ions move randomly as a result of collisions with the fluid molecules and since there are more on one side than the other there is a net flow from the more dense concentration side to the ...
... net flow from the more dense concentration side to the less. B. The ions move randomly as a result of collisions with the fluid molecules and since there are more on one side than the other there is a net flow from the more dense concentration side to the ...
Chapter 4 Reactions in Aqueous Solution 4.1 Aqueous Solutions
... 22.5 mL of 0.383 M H2SO4 are required to neutralize 20.0 mL of a KOH solution. Calculate the molarity of the KOH solution. H2SO4 + 2 KOH d K2SO4 + 2 H2O M KOH = (0.0225 L H2SO4)(0.383 mol H2SO4/L H2SO4)(2 mol KOH/ 1 mol H2SO4)/ (0.0200 mL KOH) = ...
... 22.5 mL of 0.383 M H2SO4 are required to neutralize 20.0 mL of a KOH solution. Calculate the molarity of the KOH solution. H2SO4 + 2 KOH d K2SO4 + 2 H2O M KOH = (0.0225 L H2SO4)(0.383 mol H2SO4/L H2SO4)(2 mol KOH/ 1 mol H2SO4)/ (0.0200 mL KOH) = ...
Role of Water as a Solvent
... 1. All common metal hydroxides are insoluble, except those of Group 1A(1) and the larger members of Group 2A(2)(beginning with Ca2+). 2. All common carbonates (CO32-) and phosphates (PO43-) are insoluble, except those of Group 1A(1) and NH4+. 3. All common sulfides are insoluble except those of Grou ...
... 1. All common metal hydroxides are insoluble, except those of Group 1A(1) and the larger members of Group 2A(2)(beginning with Ca2+). 2. All common carbonates (CO32-) and phosphates (PO43-) are insoluble, except those of Group 1A(1) and NH4+. 3. All common sulfides are insoluble except those of Grou ...
Data: I am writing out the question and underlining it.
... thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things l ...
... thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is made up harmful pollutants? Well when fossil fuels are burned, or maybe even things l ...
Chemical Reactions and The Mole
... By definition an AMU is 1/12th the mass of a C-12 atom. The unit of mass needs a reference point and a specific amount of matter to which all other matter can be referenced. This is a standard, as C-12 is very abundant, but this mass is very small, too small to work with. Generally, you will work wi ...
... By definition an AMU is 1/12th the mass of a C-12 atom. The unit of mass needs a reference point and a specific amount of matter to which all other matter can be referenced. This is a standard, as C-12 is very abundant, but this mass is very small, too small to work with. Generally, you will work wi ...
Unit 4/5 packet
... ___ Let’s pool together what we’ve got ___ You can have it; I really didn’t want it that much ___ I feel we both gain something from this relationship ___ Does this completed octet make me look fat? ___ As long as we stick together, we’ll be OK. ...
... ___ Let’s pool together what we’ve got ___ You can have it; I really didn’t want it that much ___ I feel we both gain something from this relationship ___ Does this completed octet make me look fat? ___ As long as we stick together, we’ll be OK. ...
Chemistry I
... Isotopes are variants of atoms of a particular chemical element, differing in the numbers of neutrons and in the atomic mass. They contain the same number of protons, but a different number of neutrons. Examples: Examples: ...
... Isotopes are variants of atoms of a particular chemical element, differing in the numbers of neutrons and in the atomic mass. They contain the same number of protons, but a different number of neutrons. Examples: Examples: ...
CHE 105 Spring 2016 Exam 3
... Select all of the true statements about quantum numbers. A. The principal quantum number, n, determines the shape of an orbital. ✓B. Energy is absorbed when an electron moves from a shell with principal quantum number n = 1 to one with n = 3. C. The angular momentum quantum number, l, determines how ...
... Select all of the true statements about quantum numbers. A. The principal quantum number, n, determines the shape of an orbital. ✓B. Energy is absorbed when an electron moves from a shell with principal quantum number n = 1 to one with n = 3. C. The angular momentum quantum number, l, determines how ...
Molecular Geometry
... • There are ___________________of electron groups around a central atom based on a maximum of six bonding electron groups though there may be more than six on very large atoms, it is very rare ...
... • There are ___________________of electron groups around a central atom based on a maximum of six bonding electron groups though there may be more than six on very large atoms, it is very rare ...
Q - PIMS
... The substance whose analysis is required for the separation of isotopes is converted into vapours. The pressure of vapours is reduced to 106—107 torr. These vapours at low pressure are allowed to enter the ionization chamber. ...
... The substance whose analysis is required for the separation of isotopes is converted into vapours. The pressure of vapours is reduced to 106—107 torr. These vapours at low pressure are allowed to enter the ionization chamber. ...
The Atomic Theory Chem 111
... ions (cations) are considered ionic. In chemical reactions the lose or gain of electrons by an atom or molecule results in a charged particle called an ion. Ion that contain more than one atom is a polyatomic ion. Example: NH4+ or NO3-. An ion with only on atom is a monatomic ion. Example: Fe3+ or S ...
... ions (cations) are considered ionic. In chemical reactions the lose or gain of electrons by an atom or molecule results in a charged particle called an ion. Ion that contain more than one atom is a polyatomic ion. Example: NH4+ or NO3-. An ion with only on atom is a monatomic ion. Example: Fe3+ or S ...
Lab 1
... Above, when adding we look for the least number of accurate places to the right. So above, all of the numbers are 3 places to the right, so our answer, 53.552, must have 3 places to the right; which makes it a 5 sig fig number. ...
... Above, when adding we look for the least number of accurate places to the right. So above, all of the numbers are 3 places to the right, so our answer, 53.552, must have 3 places to the right; which makes it a 5 sig fig number. ...
3(aq)
... • In order to identify which ions will react in an aqueous double replacement reaction (and form a solid precipitate), solubility rules/guidelines need to be reviewed. • Steps in predicting the precipitate that will form: 1. Write the reactants as they will exist upon dissolving (show their charges) ...
... • In order to identify which ions will react in an aqueous double replacement reaction (and form a solid precipitate), solubility rules/guidelines need to be reviewed. • Steps in predicting the precipitate that will form: 1. Write the reactants as they will exist upon dissolving (show their charges) ...
Chapter 4: Aqueous Reactions and Solution Stoichiometry
... Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO3)2, C6H12O6, NaCH3COO, CH3COOH. Rank the solutions in order of increasing electrical conductivity, based on the fact that the greater the number of ions in solution, the greater the conductivit ...
... Consider solutions in which 0.1 mol of each of the following compounds is dissolved in 1 L of water: Ca(NO3)2, C6H12O6, NaCH3COO, CH3COOH. Rank the solutions in order of increasing electrical conductivity, based on the fact that the greater the number of ions in solution, the greater the conductivit ...
PowerPoint
... Problem: A titration is performed between sodium hydroxide and potassium hydrogenphthalate (KHP) to standardize the base solution, by placing 50.00 mg of solid potassium hydrogenphthalate in a flask with a few drops of an indicator. A buret is filled with the base, and the initial buret reading is 0 ...
... Problem: A titration is performed between sodium hydroxide and potassium hydrogenphthalate (KHP) to standardize the base solution, by placing 50.00 mg of solid potassium hydrogenphthalate in a flask with a few drops of an indicator. A buret is filled with the base, and the initial buret reading is 0 ...
Supplemental Informaton
... (Prefix) nonmetal 1 - (Prefix) nonmetal 2 (ide) • Prefixes are indication of the number of atoms: mono-, di-, tri-, tetra-, penta-, hexa• order of naming nonmetal 1 & nonmetal (ide)2 nonmetal 1 is to the left and bottom of nonmetal 2 based on it is named first in the nomenclature scheme. Si - C - As ...
... (Prefix) nonmetal 1 - (Prefix) nonmetal 2 (ide) • Prefixes are indication of the number of atoms: mono-, di-, tri-, tetra-, penta-, hexa• order of naming nonmetal 1 & nonmetal (ide)2 nonmetal 1 is to the left and bottom of nonmetal 2 based on it is named first in the nomenclature scheme. Si - C - As ...
Writing Net Ionic Equations
... to go to completion. Unionized or partially ionized molecules give solutions that are known as nonelectrolytes or weak electrolytes. The best known nonelectrolyte is water formed in acid-base neutralization reactions. Acetic acid is an example of an acid that is primarily molecular (weak electrolyt ...
... to go to completion. Unionized or partially ionized molecules give solutions that are known as nonelectrolytes or weak electrolytes. The best known nonelectrolyte is water formed in acid-base neutralization reactions. Acetic acid is an example of an acid that is primarily molecular (weak electrolyt ...
Topic guide 9.3: Drug discovery and design
... enables it to be recognised at the binding site. Quantitative structure-activity relationship models can be used to predict the most effective ligands based on our knowledge of the pharmacophore and, as a result, we can prioritise the experimental investigation of just the most likely ligands. It is ...
... enables it to be recognised at the binding site. Quantitative structure-activity relationship models can be used to predict the most effective ligands based on our knowledge of the pharmacophore and, as a result, we can prioritise the experimental investigation of just the most likely ligands. It is ...
6 Thermodynamics
... S[Cu(s)] = 33 J/K·mol S[O2(g)] = 205 J/K·mol S[CuO(s)] = 42 J/K·mol (A) −111.5 J/mol·K (B) −93.5 J/mol·K (C) +111.5 J/mol·K (D) +93.5 J/mol·K 10. In which of the following processes is the standard entropy of reaction, ∆S°, expected to be negative? (A) (NH4)2CO3 (s) → 2 NH3 (g) + H2O (ℓ) + CO2 (g ...
... S[Cu(s)] = 33 J/K·mol S[O2(g)] = 205 J/K·mol S[CuO(s)] = 42 J/K·mol (A) −111.5 J/mol·K (B) −93.5 J/mol·K (C) +111.5 J/mol·K (D) +93.5 J/mol·K 10. In which of the following processes is the standard entropy of reaction, ∆S°, expected to be negative? (A) (NH4)2CO3 (s) → 2 NH3 (g) + H2O (ℓ) + CO2 (g ...
CP Chemistry - Final Exam Review KEY
... Compare and contrast chemical and physical changes. List signs of chemical changes. A chemical change results in a new, different substance, while a physical change does not. Chemical changes are shown with bubbling, color change, precipitate formation, temperature change and a substance “disappea ...
... Compare and contrast chemical and physical changes. List signs of chemical changes. A chemical change results in a new, different substance, while a physical change does not. Chemical changes are shown with bubbling, color change, precipitate formation, temperature change and a substance “disappea ...
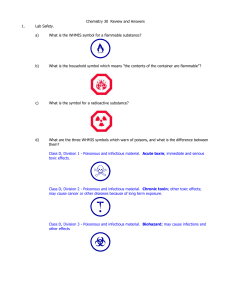
REVIEW and answers
... melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, more brittle and conduct less well, but their melting and boiling points increase (top left hand side of transition metals). ...
... melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, more brittle and conduct less well, but their melting and boiling points increase (top left hand side of transition metals). ...