____ 1. The energy required to convert a ground
... student reported a value of 38 percent. The correct value for the percentage of water in the hydrate is 51 percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the d. The crucible was not heated to constant hydrate sample to spa ...
... student reported a value of 38 percent. The correct value for the percentage of water in the hydrate is 51 percent. Which of the following is the most likely explanation for this difference? a. Strong initial heating caused some of the d. The crucible was not heated to constant hydrate sample to spa ...
GCSE - WordPress.com
... How many of each of the fundamental particles are present in the nucleus of Ne-22? What is the electron configuration of Ne-20? Why is neon a very unreactive element? Explain the meaning of the word isotope. What is the difference between the two isotopes of Neon? Calculate the relative atomic mass ...
... How many of each of the fundamental particles are present in the nucleus of Ne-22? What is the electron configuration of Ne-20? Why is neon a very unreactive element? Explain the meaning of the word isotope. What is the difference between the two isotopes of Neon? Calculate the relative atomic mass ...
Practice Qs - Unit 6a
... What is the total mass of water formed when 8 grams of hydrogen reacts completely with 64 grams of oxygen? 15. When glucose is fermented, it produces ethanol and carbon dioxide. If 60.0 grams of glucose is fermented and produces 16.5 grams of carbon dioxide gas, what mass of ethanol is produced? ...
... What is the total mass of water formed when 8 grams of hydrogen reacts completely with 64 grams of oxygen? 15. When glucose is fermented, it produces ethanol and carbon dioxide. If 60.0 grams of glucose is fermented and produces 16.5 grams of carbon dioxide gas, what mass of ethanol is produced? ...
Chapter 4
... 1: write separate half-reaction equations for oxidation and reduction 2. balance the atoms in the half reactions 3. add enough electrons to one side of each half-reaction to balance the charges 4. multiply each half-reaction by a number to make the electrons equal in both 5. add the balanced half-re ...
... 1: write separate half-reaction equations for oxidation and reduction 2. balance the atoms in the half reactions 3. add enough electrons to one side of each half-reaction to balance the charges 4. multiply each half-reaction by a number to make the electrons equal in both 5. add the balanced half-re ...
General Chemistry Discretes Test
... intervening filled electron shells, which increases the atomic radius. So trends in atomic radii result from interaction between these two competing effects. The key thing here is that the electrons in the outer shell are not very effective at shielding each other from the nuclear pull, while electr ...
... intervening filled electron shells, which increases the atomic radius. So trends in atomic radii result from interaction between these two competing effects. The key thing here is that the electrons in the outer shell are not very effective at shielding each other from the nuclear pull, while electr ...
PHYSICAL SETTING CHEMISTRY
... 8 Which type of bonding is present in a sample of an element that is malleable? (1) ionic (3) nonpolar covalent (2) metallic (4) polar covalent 9 Which atom has the greatest attraction for the electrons in a chemical bond? (1) hydrogen (3) silicon (2) oxygen (4) sulfur ...
... 8 Which type of bonding is present in a sample of an element that is malleable? (1) ionic (3) nonpolar covalent (2) metallic (4) polar covalent 9 Which atom has the greatest attraction for the electrons in a chemical bond? (1) hydrogen (3) silicon (2) oxygen (4) sulfur ...
May/Jun 16 Paper 3 - Theory (Core) QP S2
... Which one of the following values is the pH of a strongly alkaline solution? Put a ring around the correct answer. A ...
... Which one of the following values is the pH of a strongly alkaline solution? Put a ring around the correct answer. A ...
U-6 Stoichiometry Notes
... weighing, determining volume, etc. Which technique we employ is determined, in large part, by our purpose. It is also necessary, when determining which technique to use, to consider what type of measurement is easiest to make or even feasible. Consider the chemical reaction below: C12(g) + 2KI(aq) — ...
... weighing, determining volume, etc. Which technique we employ is determined, in large part, by our purpose. It is also necessary, when determining which technique to use, to consider what type of measurement is easiest to make or even feasible. Consider the chemical reaction below: C12(g) + 2KI(aq) — ...
EL Study Notes
... theory of the atom. Following a long series of experiments, Dalton suggested that: different elements have atoms which differ in mass; each element is characterised by the mass of its atoms; Four postulates describe how atoms behave: the atoms in a given element are all of the same kind; a c ...
... theory of the atom. Following a long series of experiments, Dalton suggested that: different elements have atoms which differ in mass; each element is characterised by the mass of its atoms; Four postulates describe how atoms behave: the atoms in a given element are all of the same kind; a c ...
1 - A-Level Chemistry
... d sub-shell / shell / orbitals / sub-level full (or not partially full) can only score M2 if d10 in M1 correct allow ‘full d orbital’ if d10 in M1 do not allow d block ...
... d sub-shell / shell / orbitals / sub-level full (or not partially full) can only score M2 if d10 in M1 correct allow ‘full d orbital’ if d10 in M1 do not allow d block ...
Homework Booklet [4,S]
... How many of each of the fundamental particles are present in the nucleus of Ne-22? What is the electron configuration of Ne-20? Why is neon a very unreactive element? Explain the meaning of the word isotope. What is the difference between the two isotopes of Neon? Calculate the relative atomic mass ...
... How many of each of the fundamental particles are present in the nucleus of Ne-22? What is the electron configuration of Ne-20? Why is neon a very unreactive element? Explain the meaning of the word isotope. What is the difference between the two isotopes of Neon? Calculate the relative atomic mass ...
Problem 1: “A brief history” of life in the universe
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
Problem 1: A brief history of life in the universe
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
Problem 1: “A brief history” of life in the universe
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
... Problem 6: Discovery of the noble gases Molecules such as H2, N2, O2, CO2, and CH4 in Problem 5 are formed through chemical bonding of atoms. Even though valency was known in the 19th century, the underlying principle behind chemical bonding had not been understood for a long time. Ironically, the d ...
Reaction Rate review questions
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
answers to part a of the national high school
... to 2005 exams (questions and answers) before attempting the 2006 examination. Students should try to do the questions in Part A of the 2006 Examination on their own, and then compare their answers with the solutions given below. They should not be put off by the length of the explanations, which do ...
... to 2005 exams (questions and answers) before attempting the 2006 examination. Students should try to do the questions in Part A of the 2006 Examination on their own, and then compare their answers with the solutions given below. They should not be put off by the length of the explanations, which do ...
Chapter 17: An Introduction to Organic Chemistry, Biochemistry, and
... into the millions. Fortunately, the task of studying them is not so daunting as their number would suggest, because organic compounds can be categorized according to structural similarities that lead to similarities in the compounds’ important properties. For example, you discovered in Section 3.3 t ...
... into the millions. Fortunately, the task of studying them is not so daunting as their number would suggest, because organic compounds can be categorized according to structural similarities that lead to similarities in the compounds’ important properties. For example, you discovered in Section 3.3 t ...
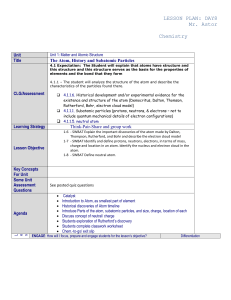
lesson plan - cloudfront.net
... Teacher can use What Do You See?/What Do you think? Active Chem 1.3 p 15. Students can use worksheet to guide them through activity. Active Chem 1.3 Investigate: One way to think about an atom is to imagine trying to isolate it from a larger number of atoms. Distribute the same size squares of alu ...
... Teacher can use What Do You See?/What Do you think? Active Chem 1.3 p 15. Students can use worksheet to guide them through activity. Active Chem 1.3 Investigate: One way to think about an atom is to imagine trying to isolate it from a larger number of atoms. Distribute the same size squares of alu ...
answers to part a of the canadian chemistry
... answer in MB, compared with only 37% in AB). It requires students to choose the atom with the smallest atomic radius, which means that they need to know (1) that periods that are lower in the periodic table have larger atoms in them (because larger atoms have more electrons and some of these will be ...
... answer in MB, compared with only 37% in AB). It requires students to choose the atom with the smallest atomic radius, which means that they need to know (1) that periods that are lower in the periodic table have larger atoms in them (because larger atoms have more electrons and some of these will be ...
Efficient Phosphodiester Hydrolysis by
... Reactivity. Kinetic experiments for the hydrolysis of the model substrate BDNPP were monitored spectrophotometrically for the absorbance increase at 400 nm due to the formation of 2,4-dinitrophenolate over time, under conditions of excess complex at 25 °C, and the reaction mixtures were monitored un ...
... Reactivity. Kinetic experiments for the hydrolysis of the model substrate BDNPP were monitored spectrophotometrically for the absorbance increase at 400 nm due to the formation of 2,4-dinitrophenolate over time, under conditions of excess complex at 25 °C, and the reaction mixtures were monitored un ...
Chemistry (English) Grade 11 and 12
... similar number of carbon atoms are higher than the boiling points of both the alkanes and the aldehyde / ketones. (You can see this because the lowest (or bottom) line on the graph is that of the alkanes and the top line is that of the alcohols). The graph is simply a way of showing this trend in t ...
... similar number of carbon atoms are higher than the boiling points of both the alkanes and the aldehyde / ketones. (You can see this because the lowest (or bottom) line on the graph is that of the alkanes and the top line is that of the alcohols). The graph is simply a way of showing this trend in t ...









![Homework Booklet [4,S]](http://s1.studyres.com/store/data/010355871_1-63c750e3d1b58eaaebbb3f5d45651c44-300x300.png)