Utah - Wavefunction, Inc.
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
Document
... An unknown white solid is discovered on the lab counter in room 2101. Miss Allen wants to know if it is ionic or covalent. Describe how you could use its properties to determine if it is ionic or covalent. Be sure to use at least 3 specific examples of properties (3 marks) ...
... An unknown white solid is discovered on the lab counter in room 2101. Miss Allen wants to know if it is ionic or covalent. Describe how you could use its properties to determine if it is ionic or covalent. Be sure to use at least 3 specific examples of properties (3 marks) ...
Equilibrium Part 2
... a system at equilibrium. The system attempts to remove added heat by using it up in the forward reaction (endothermic reaction). The equilibrium position shifts towards the right (products). The concentration of NO2 increases and the concentration of N2O4 decreases. We can also think of adding heat ...
... a system at equilibrium. The system attempts to remove added heat by using it up in the forward reaction (endothermic reaction). The equilibrium position shifts towards the right (products). The concentration of NO2 increases and the concentration of N2O4 decreases. We can also think of adding heat ...
Chemistry EOC Review
... 59) You want to dissolve as much carbon dioxide as possible into a 2 liter bottle of Mountain Dew. What conditions of temperature (high or low) and pressure (high or low) should you use to dissolve the maximum amount of CO2? Would your answers be the same if you were trying to dissolve as much sugar ...
... 59) You want to dissolve as much carbon dioxide as possible into a 2 liter bottle of Mountain Dew. What conditions of temperature (high or low) and pressure (high or low) should you use to dissolve the maximum amount of CO2? Would your answers be the same if you were trying to dissolve as much sugar ...
(null): 110.ReactionsIntro
... b) KE transformed into chem PE 3) Do reduced version of Zn & HCl: one zinc pellet in test tube plus a few ml of HCl. While bubbling discuss where energy is stored and where it goes 4) Return to reaction, have Ss feel test tube (warm!) & decide if reaction followed Option 1 or 2 d. “Re-arrange” colli ...
... b) KE transformed into chem PE 3) Do reduced version of Zn & HCl: one zinc pellet in test tube plus a few ml of HCl. While bubbling discuss where energy is stored and where it goes 4) Return to reaction, have Ss feel test tube (warm!) & decide if reaction followed Option 1 or 2 d. “Re-arrange” colli ...
Chemical Reactions.
... a physical change n Reactants: chemicals that react n Products: chemicals that are formed n e.x. sodium + oxygen à sodium oxide Na(s) + O2(g) à Na2O(s) reactants ...
... a physical change n Reactants: chemicals that react n Products: chemicals that are formed n e.x. sodium + oxygen à sodium oxide Na(s) + O2(g) à Na2O(s) reactants ...
Chemistry 123: Physical and Organic Chemistry
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
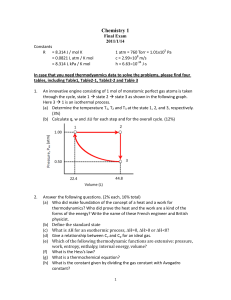
Chemistry 1
... (b) Define the standard state (c) What is H for an exothermic process, H=0, H>0 or H<0? (d) Give a relationship between Cv and Cp for an ideal gas. (e) Which of the following thermodynamic functions are extensive: pressure, work, entropy, enthalpy, internal energy, volume? (f) What is the Hess’s ...
... (b) Define the standard state (c) What is H for an exothermic process, H=0, H>0 or H<0? (d) Give a relationship between Cv and Cp for an ideal gas. (e) Which of the following thermodynamic functions are extensive: pressure, work, entropy, enthalpy, internal energy, volume? (f) What is the Hess’s ...
AP Chapter Five Outline
... When ionic compounds dissolve in water, they dissociate into ions surrounded by water molecules. An ionic compound that completely dissolves into ions is a strong electrolyte. A. Exchange Reactions: AB + CD AD + CB 1. If both reactants and products are water-soluble compounds, then no overall re ...
... When ionic compounds dissolve in water, they dissociate into ions surrounded by water molecules. An ionic compound that completely dissolves into ions is a strong electrolyte. A. Exchange Reactions: AB + CD AD + CB 1. If both reactants and products are water-soluble compounds, then no overall re ...
Experiment 7
... 5. Pipette 10 ml aliquots of the solutions in the water bath (from Part 1) using the cotton filter attached to the base of the pipette and titrate with the standardized NaOH solution using phenolphthalein as the indicator. 6. For each of the five solutions prepared, a minimum of three titrations sho ...
... 5. Pipette 10 ml aliquots of the solutions in the water bath (from Part 1) using the cotton filter attached to the base of the pipette and titrate with the standardized NaOH solution using phenolphthalein as the indicator. 6. For each of the five solutions prepared, a minimum of three titrations sho ...
Chapter 18 review
... ____ 22. What is the overall rate order of the following reaction? A + 2B → C + D a. zero b. first c. second d. third ____ 23. In a first-order reaction, how does the rate change if the concentration of the reactant decreases to one-third its original value? a. The rate decreases by a factor of one- ...
... ____ 22. What is the overall rate order of the following reaction? A + 2B → C + D a. zero b. first c. second d. third ____ 23. In a first-order reaction, how does the rate change if the concentration of the reactant decreases to one-third its original value? a. The rate decreases by a factor of one- ...
Part I - American Chemical Society
... Part I must be entered on a Scantron answer sheet to be scored. Be sure to write your name on the answer sheet, an ID number is already entered for you. Make a record of this ID number because you will use the same number on Parts II and III. Each item in Part I consists of a question or an incomple ...
... Part I must be entered on a Scantron answer sheet to be scored. Be sure to write your name on the answer sheet, an ID number is already entered for you. Make a record of this ID number because you will use the same number on Parts II and III. Each item in Part I consists of a question or an incomple ...
I have put this in the format of the 1984 exam
... of the steps in the mechanism of the reaction (C) substance B is not involved in the rate-determining step of the mechanism, but is involved in subsequent steps (D) substance B is probably a catalyst, and as such, its effect on the rate of the reaction does not depend on its concentration (E) the re ...
... of the steps in the mechanism of the reaction (C) substance B is not involved in the rate-determining step of the mechanism, but is involved in subsequent steps (D) substance B is probably a catalyst, and as such, its effect on the rate of the reaction does not depend on its concentration (E) the re ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.