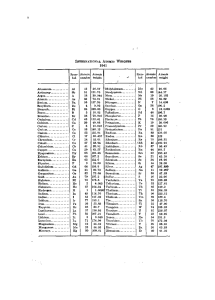
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, who each year come better prepared for the study of physical chemistry. A certain amount of elasticity is needed in the amount of labor ...
... chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, who each year come better prepared for the study of physical chemistry. A certain amount of elasticity is needed in the amount of labor ...
Chapter 4 - KFUPM Faculty List
... Work done on the system (gas) increases the energy of the system: w = + 85 J Heat given off to the surroundings is energy lost by the system: q = -32 J Thus ΔE = q + w = (-32 + 85) J = +53 J ...
... Work done on the system (gas) increases the energy of the system: w = + 85 J Heat given off to the surroundings is energy lost by the system: q = -32 J Thus ΔE = q + w = (-32 + 85) J = +53 J ...
Rutile titanium dioxide nanoparticles and ordered acicular
... crystallites are joined, i.e., assembled, into a cluster such that the opposite ends of each of the crystallites extend, or fan, outwardly in the general shape of a nano-sized ?ower bouquet or a funnel. The funnel-shaped structures have a diameter in the range of 50 nm and a height in the range of f ...
... crystallites are joined, i.e., assembled, into a cluster such that the opposite ends of each of the crystallites extend, or fan, outwardly in the general shape of a nano-sized ?ower bouquet or a funnel. The funnel-shaped structures have a diameter in the range of 50 nm and a height in the range of f ...
Supplemental information
... prepared at a particle concentration of 100 mg·L-1 as Fe but with various concentration of CMC ranging from 0 to 0.161 wt %. Based on the results, this study focused on fully stabilized HFO nanoparticle suspensions, which were prepared at a final concentration of 100 mg·L-1 as Fe and 0.064 wt % CMC ...
... prepared at a particle concentration of 100 mg·L-1 as Fe but with various concentration of CMC ranging from 0 to 0.161 wt %. Based on the results, this study focused on fully stabilized HFO nanoparticle suspensions, which were prepared at a final concentration of 100 mg·L-1 as Fe and 0.064 wt % CMC ...
Chemistry - Department of Education and Skills
... Physics and Chemistry. Evaluations of this programme have shown that it has had an important impact on the participation levels in Physics and Chemistry among girls in the target schools. This present handbook is the most recent example of the work of these worthwhile Intervention Projects. However, ...
... Physics and Chemistry. Evaluations of this programme have shown that it has had an important impact on the participation levels in Physics and Chemistry among girls in the target schools. This present handbook is the most recent example of the work of these worthwhile Intervention Projects. However, ...
View/Open
... The heat of a reaction is simply the amount of heat absorbed or evolved in the reaction. We also know that the amount of heat absorbed or evolved at constant temperature and pressure is called enthalpy. Therefore the amount of heat change during a reaction at constant temperature and pressure may al ...
... The heat of a reaction is simply the amount of heat absorbed or evolved in the reaction. We also know that the amount of heat absorbed or evolved at constant temperature and pressure is called enthalpy. Therefore the amount of heat change during a reaction at constant temperature and pressure may al ...
Problem 1-2
... Another possibility to find reaction orders and rate constants is the so called method of isolation combined with the method of initial rates. The data in Tab. 3 is measured for reaction (4) at room temperature. Tab 3. Test series of initial rates and concentrations or reaction (4) c(H3AsO3) ...
... Another possibility to find reaction orders and rate constants is the so called method of isolation combined with the method of initial rates. The data in Tab. 3 is measured for reaction (4) at room temperature. Tab 3. Test series of initial rates and concentrations or reaction (4) c(H3AsO3) ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined Stoichiometry ...
... – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been determined Stoichiometry ...
Regents Chemistry Review - New York Science Teacher
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
CHAPTER 4 - Myschoolpages.com
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
... Nonelectrolytes are not dissociated into ions in solution Extent of dissolution does not dictate strong or weak electrolyte solution (i.e., HC2H3O2 is very soluble but is a weak electrolyte while Ba(OH)2 is only slightly soluble is a strong electrolyte) ...
Experiments in General Chemistry: Featuring MeasureNet
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
... Chemists observe matter by determining, measuring, and monitoring physical and chemical properties of matter. A property is any characteristic that can be used to describe matter (e.g., size, color, mass, density, solubility, etc.). In this experiment, we will determine the density of liquids and so ...
Chapter 8 - Chemical Equations and Reactions
... • A chemical reaction is the process by which one or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mas ...
... • A chemical reaction is the process by which one or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mas ...
Chem13-14PrecipABNeut
... write your work when solving the problems in these lessons. A notebook that has graphpaper as its pages will be especially helpful. Choosing a Calculator: As you do problems in these lessons (and assigned homework) that require a calculator, use the same calculator that you will be allowed to use du ...
... write your work when solving the problems in these lessons. A notebook that has graphpaper as its pages will be especially helpful. Choosing a Calculator: As you do problems in these lessons (and assigned homework) that require a calculator, use the same calculator that you will be allowed to use du ...
44. Find рН of formic acid solution with mass percent ω=5
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.