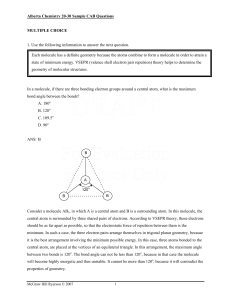
Vol 1 No 2.10
... state, R (J.mol-1. K-1) is the universal gas constant, µ0 (J.mol-1) is the chemical potential at the pressure P0 and temperature T, and ϑ (m3/mol) is the partial molar volume of the species. According to the Eq. (2), under steady state flow, the chemical potential decreases linearly, and the concent ...
... state, R (J.mol-1. K-1) is the universal gas constant, µ0 (J.mol-1) is the chemical potential at the pressure P0 and temperature T, and ϑ (m3/mol) is the partial molar volume of the species. According to the Eq. (2), under steady state flow, the chemical potential decreases linearly, and the concent ...
CLUE - virtual laboratories
... Einstein and Infeld's “The Evolution of Physics” present science in a logical and engaging manner; they are both interesting and stimulating to read. Unfortunately, this is quite different from the style found in most textbooks. So what is missing from the Bryson and Einstein and Infeld books that m ...
... Einstein and Infeld's “The Evolution of Physics” present science in a logical and engaging manner; they are both interesting and stimulating to read. Unfortunately, this is quite different from the style found in most textbooks. So what is missing from the Bryson and Einstein and Infeld books that m ...
Decrease = stress More Fe(OH) 2 dissolves in response Solubility
... Why Study Solubility Equilibria? • To understand precipitation/dissolution processes in nature, and how to exploit precipitation/dissolution processes for useful purposes, we need to look at the quantitative aspects of solubility and solubility equilibria. ...
... Why Study Solubility Equilibria? • To understand precipitation/dissolution processes in nature, and how to exploit precipitation/dissolution processes for useful purposes, we need to look at the quantitative aspects of solubility and solubility equilibria. ...
Precipitation of salts in freezing seawater and ozone depletion
... aerosol particles whereas in (iii) bromine would be released from the snow pack into the atmosphere as photolysable gas phase compound (e.g. Br2 or BrCl). The key commonality of the three processes is that a physical separation of the brine and all precipitates is occuring. It is currently believed ...
... aerosol particles whereas in (iii) bromine would be released from the snow pack into the atmosphere as photolysable gas phase compound (e.g. Br2 or BrCl). The key commonality of the three processes is that a physical separation of the brine and all precipitates is occuring. It is currently believed ...
Theoretical Study of Gas-Phase Reactions of Fe(CO)5 with OH
... Revision of the homogeneously Fe(CO)5-catalyzed water gas shift reaction in the gas phase has been performed by means of quantum chemical calculations using gradient-corrected density functional theory (B3LYP) and ab initio methods at the CCSD(T) level. The classically assumed reaction path has been ...
... Revision of the homogeneously Fe(CO)5-catalyzed water gas shift reaction in the gas phase has been performed by means of quantum chemical calculations using gradient-corrected density functional theory (B3LYP) and ab initio methods at the CCSD(T) level. The classically assumed reaction path has been ...
Chemistry of Riming: The Retention of Organic and Inorganic
... concentrations used in the experiments were approximately one order of magnitude higher than those found in cloud water. However, the resulting pH values were in a range which is typically found in cloud water, i.e., from 3.5 – 5.3 (Löflund et al., 2002). The solutions, containing a single substance ...
... concentrations used in the experiments were approximately one order of magnitude higher than those found in cloud water. However, the resulting pH values were in a range which is typically found in cloud water, i.e., from 3.5 – 5.3 (Löflund et al., 2002). The solutions, containing a single substance ...
evaluation copy
... 1. MODELING CHEMISTRY / STUDENT HANDOUT 18. Use the remaining solutions in Beakers A–D to repeat the reactions carried out above by combining the solutions in Beakers A and B and making measurements, and then combining the solutions in Beakers C and D and making measurements. Record observations of ...
... 1. MODELING CHEMISTRY / STUDENT HANDOUT 18. Use the remaining solutions in Beakers A–D to repeat the reactions carried out above by combining the solutions in Beakers A and B and making measurements, and then combining the solutions in Beakers C and D and making measurements. Record observations of ...
Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic
... • Chemical Reactions do not always go the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
... • Chemical Reactions do not always go the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
TRIPURA UNIVERSITY SURYAMANINAGAR SYLLABUS FOR B.Sc THREE-YEAR DEGREE (GENERAL AND HONOURS) COURSE
... f) Two questions of 15 marks each are to be set from each unit, out of which one question is to be answered . Each question of 15 marks may be divided into three or more parts having a maximum of 8 marks for a part. Unit-I: The Gaseous and crystalline states of matter The Gaseous state: Gas laws; po ...
... f) Two questions of 15 marks each are to be set from each unit, out of which one question is to be answered . Each question of 15 marks may be divided into three or more parts having a maximum of 8 marks for a part. Unit-I: The Gaseous and crystalline states of matter The Gaseous state: Gas laws; po ...
Question Bank for Pre Board Exam(XII Chemistry)
... 41.Name the crystal defect which lowers the density of an ionic crystal. 42 What makes the crystal of KCl sometimes appear violet? 43 Which point defect in ionic crystal does not alter the density of the relevant solid? 44.Name one solid in which both Frenkel and Schottky defects occur. 45.Which typ ...
... 41.Name the crystal defect which lowers the density of an ionic crystal. 42 What makes the crystal of KCl sometimes appear violet? 43 Which point defect in ionic crystal does not alter the density of the relevant solid? 44.Name one solid in which both Frenkel and Schottky defects occur. 45.Which typ ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.