The Atom
... alphabet to the language of molecules. To make molecules, you must have elements. Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... alphabet to the language of molecules. To make molecules, you must have elements. Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
The Periodic Table
... • What is it? • The energy release when an electron is added to an atom. Most favorable toward NE corner of PT since these atoms have a great affinity (“love and happiness”) for e-. (Think of “Jumping for Joy”!) • What happens down a group? • Decreases; since the electrons are further from the nucle ...
... • What is it? • The energy release when an electron is added to an atom. Most favorable toward NE corner of PT since these atoms have a great affinity (“love and happiness”) for e-. (Think of “Jumping for Joy”!) • What happens down a group? • Decreases; since the electrons are further from the nucle ...
Chapter 2
... Metalloids border the stair-step line (with the exception of Al and Po). Metals are on the left side of the chart. The subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound. Molecular compounds are composed of molecules and almos ...
... Metalloids border the stair-step line (with the exception of Al and Po). Metals are on the left side of the chart. The subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound. Molecular compounds are composed of molecules and almos ...
20161018145157
... What is the most stable electron configuration? o When all electrons are in the lowest possible energy levels (ground state) What do scientists use the electron cloud model for? o To describe the possible locations of electrons around the nucleus. ...
... What is the most stable electron configuration? o When all electrons are in the lowest possible energy levels (ground state) What do scientists use the electron cloud model for? o To describe the possible locations of electrons around the nucleus. ...
Chapter 3 - mrgoosby
... The inner ring, #1, can only have 2 electrons The second ring can have up to 8 electrons The third ring can have up to 18 electrons The fourth ring can have up to 32 electrons All rings up to ring #7, the last ring, can have up to 32 electrons KEY VOCAB: Energy Level – the rings containing electrons ...
... The inner ring, #1, can only have 2 electrons The second ring can have up to 8 electrons The third ring can have up to 18 electrons The fourth ring can have up to 32 electrons All rings up to ring #7, the last ring, can have up to 32 electrons KEY VOCAB: Energy Level – the rings containing electrons ...
V. Chemical reactions
... b. Which elements have two valence electrons? Column 2 c. Which elements have three valence electrons? Column 13 d. Which elements have four valence electrons? Column 14 e. Which elements have five valence electrons? Column 15 f. Which elements have six valence electrons? Column 16 g. Which elements ...
... b. Which elements have two valence electrons? Column 2 c. Which elements have three valence electrons? Column 13 d. Which elements have four valence electrons? Column 14 e. Which elements have five valence electrons? Column 15 f. Which elements have six valence electrons? Column 16 g. Which elements ...
CHEM 1411 CHAPTER 2
... Atomic number is taken as the basis for the arrangement of the elements, because when the elements are arranged in the increasing order of their atomic numbers, elements with similar properties repeat after a regular interval. This is called Periodic law The horizontal rows are called periods and th ...
... Atomic number is taken as the basis for the arrangement of the elements, because when the elements are arranged in the increasing order of their atomic numbers, elements with similar properties repeat after a regular interval. This is called Periodic law The horizontal rows are called periods and th ...
Introduction to Chemistry for Coach Keith`s Biology
... Organisms eat plants, break down the sugars, and release energy along with CO 2 & H2O Exergonic reactions involve a net release of energy; while endergonic reactions involve a net absorption of energy Energy must be added to the reactants for most chemical reactions to occur; called activation energ ...
... Organisms eat plants, break down the sugars, and release energy along with CO 2 & H2O Exergonic reactions involve a net release of energy; while endergonic reactions involve a net absorption of energy Energy must be added to the reactants for most chemical reactions to occur; called activation energ ...
Atomic Theory - Boone County Schools
... All atoms of the same element have the same mass, and atoms of different elements have different masses. Compounds contain atoms of more than one element. In a particular compound, atoms of different elements always combine in the same way. ...
... All atoms of the same element have the same mass, and atoms of different elements have different masses. Compounds contain atoms of more than one element. In a particular compound, atoms of different elements always combine in the same way. ...
- Elliott Hudson College
... Chemistry Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number o ...
... Chemistry Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number o ...
Review Worksheet
... For each set of elements indicate which one of the four is the answer. 1) B ...
... For each set of elements indicate which one of the four is the answer. 1) B ...
Chapter 4 Review Worksheet. Name
... 3. Use the following information to determine the atomic mass of chlorine. Two isotopes are known: chlorine-35 (mass = 34.97 amu) and chlorine-37 (mass = 36.97 amu). The relative abundance’s are 75.4% and 24.6%, respectively. ...
... 3. Use the following information to determine the atomic mass of chlorine. Two isotopes are known: chlorine-35 (mass = 34.97 amu) and chlorine-37 (mass = 36.97 amu). The relative abundance’s are 75.4% and 24.6%, respectively. ...
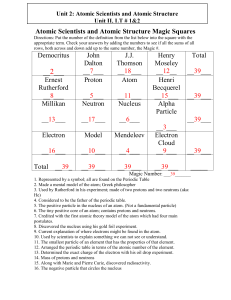
Atomic Scientists and Atomic Structure Magic Squares
... 5. The positive particle in the nucleus of an atom. (Not a fundamental particle) 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9 ...
... 5. The positive particle in the nucleus of an atom. (Not a fundamental particle) 6. The tiny positive core of an atom; contains protons and neutrons. 7. Credited with the first atomic theory model of the atom which had four main postulates. 8. Discovered the nucleus using his gold foil experiment. 9 ...
Atoms, Elements, and Ions
... What: Solid Sphere Model (1st atomic theory) •Elements are made up of indivisible particles called atoms •Each element was composed of the same kind of atoms. •Different elements were composed of different kinds of atoms. •Compounds are composed of atoms in specific ratios. •Atoms are not created or ...
... What: Solid Sphere Model (1st atomic theory) •Elements are made up of indivisible particles called atoms •Each element was composed of the same kind of atoms. •Different elements were composed of different kinds of atoms. •Compounds are composed of atoms in specific ratios. •Atoms are not created or ...
Topic 13 – 14.1
... 14.1 How atoms of various elements are different The atoms of different elements contain different numbers of protons in the nucleus. Because the number of protons is so important, it is called the atomic number. ...
... 14.1 How atoms of various elements are different The atoms of different elements contain different numbers of protons in the nucleus. Because the number of protons is so important, it is called the atomic number. ...
The Atomic Nature of Matter
... • There are about 10 to the 23 atoms in a gram of water (thimbleful) • Atoms are always moving • It takes 6 years for one of your exhaled breaths to mix evenly with the atmosphere ...
... • There are about 10 to the 23 atoms in a gram of water (thimbleful) • Atoms are always moving • It takes 6 years for one of your exhaled breaths to mix evenly with the atmosphere ...
Atomic Number - Manhasset Schools
... 8 protons, 6 electrons, and 6 neutrons? 5) How many electrons does C-14 have? ...
... 8 protons, 6 electrons, and 6 neutrons? 5) How many electrons does C-14 have? ...
Subatomic notes - Chemistry R: 4(AE) 5(A,C)
... Elements differ in their number of protons and therefore in the amount of positive charge their nuclei possess. ...
... Elements differ in their number of protons and therefore in the amount of positive charge their nuclei possess. ...
Atoms, Molecules, and Ions Chapter 2 Handout 1 The Atom Dalton`s
... For Full Atomic Theory Read: Section 2.2 in Textbook Modern Knowledge of Atomic Structure ...
... For Full Atomic Theory Read: Section 2.2 in Textbook Modern Knowledge of Atomic Structure ...
Another look at chemical reactions HYDROGEN PEROXIDE WATER
... Another look at chemical reactions The decomposition of hydrogen peroxide over time (or when poured over a cut) works like this: OXYGEN HYDROGEN WATER GAS PEROXIDE H O ...
... Another look at chemical reactions The decomposition of hydrogen peroxide over time (or when poured over a cut) works like this: OXYGEN HYDROGEN WATER GAS PEROXIDE H O ...
Models of the Atom: A Historical perspective
... • Most particles passed through • So, atoms are mostly empty space ...
... • Most particles passed through • So, atoms are mostly empty space ...