Compounds Booklet Companion New 2013
... The atomic number is typically found in the top left corner of each element square. It shows how many protons are in one atom of that element. Atoms have a neutral charge, therefore the number of electrons equals the number of protons. As you move across the periodic table from left to right the ato ...
... The atomic number is typically found in the top left corner of each element square. It shows how many protons are in one atom of that element. Atoms have a neutral charge, therefore the number of electrons equals the number of protons. As you move across the periodic table from left to right the ato ...
matter and its reactivity. Objects in the universe are composed of
... 3.1a Substances have characteristic properties. Some of these properties include color, odor, phase, density, solubility, heat and electrical conductivity, and boiling and freezing points. 3.1b Solubility can be affected by the nature of the solute and solvent, temperature, and pressure. The rate of ...
... 3.1a Substances have characteristic properties. Some of these properties include color, odor, phase, density, solubility, heat and electrical conductivity, and boiling and freezing points. 3.1b Solubility can be affected by the nature of the solute and solvent, temperature, and pressure. The rate of ...
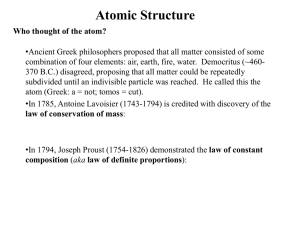
John Dalton`s atomic theories were introduced in 18 hundreds
... John Dalton wrote his first table of atomic weights in his daily journal. Two years after he developed his atomic weights, he put them in a book called "A New System of Chemical Philosophy.” In it he was the first to discover that elements should be identified with symbols. However, only 3 or 4 page ...
... John Dalton wrote his first table of atomic weights in his daily journal. Two years after he developed his atomic weights, he put them in a book called "A New System of Chemical Philosophy.” In it he was the first to discover that elements should be identified with symbols. However, only 3 or 4 page ...
10th Grade Chemistry X (TJ) GRADE(S)/LEVELS SUBJECT Power
... properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. LT 1 Predict the properties of elements in the periodic table, based on their position in a group and period. LT 2 Predict periodic table trends (e. ...
... properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. LT 1 Predict the properties of elements in the periodic table, based on their position in a group and period. LT 2 Predict periodic table trends (e. ...
2010 Physical Science Comprehensive Test REVIEW Ch 0.3 Sig
... 38. Be able to identify the atomic number, mass number, and stable isotopes. Your periodic table will not have a key on it. Such as: What is the atomic number of phosphorus? Such as: What is the mass number of K-41? Such as: K-41 is stable, but K-40 is not 42. Many models have been developed to expl ...
... 38. Be able to identify the atomic number, mass number, and stable isotopes. Your periodic table will not have a key on it. Such as: What is the atomic number of phosphorus? Such as: What is the mass number of K-41? Such as: K-41 is stable, but K-40 is not 42. Many models have been developed to expl ...
GEO143_lab_3_atoms_m..
... in the physical world. The makeup of the entire world is dependent on the configuration of individual atoms. Understanding the chemistry and physics of the atom helps us understand our world. ...
... in the physical world. The makeup of the entire world is dependent on the configuration of individual atoms. Understanding the chemistry and physics of the atom helps us understand our world. ...
Pearson Prentice Hall Physical Science: Concepts in Action
... Q: What subatomic particle could be substituted for atomic number? Q: What 2 values represent mass#? ...
... Q: What subatomic particle could be substituted for atomic number? Q: What 2 values represent mass#? ...
2.4 The Periodic Table
... The periodic table can be divided into blocks of elements according to the subshell filled last. • An s-Block element is a main group element that results from the filling of an s orbital. • A p-Block element is a main group element that results from the filling of p orbitals. • A d-Block element is ...
... The periodic table can be divided into blocks of elements according to the subshell filled last. • An s-Block element is a main group element that results from the filling of an s orbital. • A p-Block element is a main group element that results from the filling of p orbitals. • A d-Block element is ...
Unit 16 Worksheet - Jensen Chemistry
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
... 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus d. are released by the atom 2. Helium was discovered on the sun in 1868, almost 30 years before it was ...
Day 23 How Atoms Differ - WaylandHighSchoolChemistry
... Schrödinger used lots and lots of fancy mathematics, and made a model of the atom based on quantum mechanics. It has orbitals and those are based on probability. The atom is a fuzzy blob of ...
... Schrödinger used lots and lots of fancy mathematics, and made a model of the atom based on quantum mechanics. It has orbitals and those are based on probability. The atom is a fuzzy blob of ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
The Atom
... Dates (some may not have dates) Important Peoples Names What they found/discovered/believed to be true The experiment they did Picture of the model they developed (if there is one) ...
... Dates (some may not have dates) Important Peoples Names What they found/discovered/believed to be true The experiment they did Picture of the model they developed (if there is one) ...
I CAN write Chemical formulas
... 1. Write the oxidation number above each element. 2. Cross the oxidation numbers and write the oxidation number (without plus or minus) of one element as the subscript of the other element. 3. Reduce the subscripts (number of atoms) to their simplest form, if needed. WHAT IS THE CHEMICAL FORMULA FO ...
... 1. Write the oxidation number above each element. 2. Cross the oxidation numbers and write the oxidation number (without plus or minus) of one element as the subscript of the other element. 3. Reduce the subscripts (number of atoms) to their simplest form, if needed. WHAT IS THE CHEMICAL FORMULA FO ...
Basics Of Chemistry
... 3. Atoms of the same element are identical in size, mass and other properties. ...
... 3. Atoms of the same element are identical in size, mass and other properties. ...
CHAPTER 3, ATOMS: THE BUILDING BLOCKS OF MATTER
... 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be subdivided, created, or destroyed. 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds ...
... 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be subdivided, created, or destroyed. 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds ...
Atomic Structure - OCPS TeacherPress
... The number of valence electrons in an atom will determine if an element will allow electricity to flow. The ability of an atom to draw electrons to itself (away from its neighbors) is called Electronegativity. ...
... The number of valence electrons in an atom will determine if an element will allow electricity to flow. The ability of an atom to draw electrons to itself (away from its neighbors) is called Electronegativity. ...
ch4atomicstucture - Duplin County Schools
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Atomic Size - ThinkChemistry
... on going down a column in the periodic table and also on going across a row. Going Down a Group (column): The size of an atom increases going down a group. This is because on going down the group from one element to the next, an electron energy level is added each time. Going Across a Period (row): ...
... on going down a column in the periodic table and also on going across a row. Going Down a Group (column): The size of an atom increases going down a group. This is because on going down the group from one element to the next, an electron energy level is added each time. Going Across a Period (row): ...
Topic 3 : Atoms and the Periodic Table Isotopes X
... Elements could be classified (grouped together in families) in various ways e.g. solids, liquids and gases or metals and non-metals. The best way is to place them in groups having similar chemical properties (The Periodic Table). The chemical properties of elements depend on the number of unpaired o ...
... Elements could be classified (grouped together in families) in various ways e.g. solids, liquids and gases or metals and non-metals. The best way is to place them in groups having similar chemical properties (The Periodic Table). The chemical properties of elements depend on the number of unpaired o ...
AP CHEMISTRY SUMMER ASSIGNMENT AP Chemistry is a
... conductors, shiny, malleable and ductile. They are solids except for liquid mercury. Nonmetals – elements located to the right of the staircase, plus Hydrogen; they share or gain electrons. Nonmetals can be solids, liquid (Br2) or gases. They are brittle and are poor conductors of electricity. Metal ...
... conductors, shiny, malleable and ductile. They are solids except for liquid mercury. Nonmetals – elements located to the right of the staircase, plus Hydrogen; they share or gain electrons. Nonmetals can be solids, liquid (Br2) or gases. They are brittle and are poor conductors of electricity. Metal ...
Chp 4 slideshow notes - Lower Cape May Regional School District
... substance is called its molar mass. Example, one mole of lithium has a mass of 6.941 g. Because mass and amount of a substance are related, it is possible to convert moles to grams and vice ...
... substance is called its molar mass. Example, one mole of lithium has a mass of 6.941 g. Because mass and amount of a substance are related, it is possible to convert moles to grams and vice ...
Lecture 2
... • While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have more than one naturally occurring isotope: ...
... • While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have more than one naturally occurring isotope: ...
SS18A - Atoms, Isotopes and Ions
... 1. Look up bromine on the periodic table. What is the most common isotope of bromine? 2. Look up potassium on the periodic table. How many neutrons does the most common isotope of potassium have? ...
... 1. Look up bromine on the periodic table. What is the most common isotope of bromine? 2. Look up potassium on the periodic table. How many neutrons does the most common isotope of potassium have? ...