Nature of Atoms Atomic Structure Atomic number Atomic mass
... ◦ Cl atom gains an electron to become Cl– ◦ Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound ...
... ◦ Cl atom gains an electron to become Cl– ◦ Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound ...
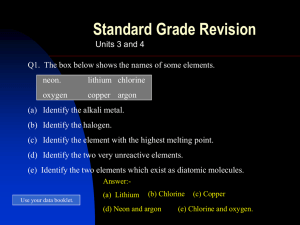
Topic 3&4 Atoms and the per.table
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
2014MSC(ORGANIC(CHEMISTRY!
... ! Polar!covalent!bonds!are!formed!through!the!sharing!of!electrons!between!neutral!atoms!–! it!is!polar!where!the!electrons!are!attracted!stronger!to!one!atom!over!the!other.!! ! Therefore,!the!electron!distribution!between!the!atoms!is!not!symmetrical,!and!atoms!have! a!partial!negative!or!positive ...
... ! Polar!covalent!bonds!are!formed!through!the!sharing!of!electrons!between!neutral!atoms!–! it!is!polar!where!the!electrons!are!attracted!stronger!to!one!atom!over!the!other.!! ! Therefore,!the!electron!distribution!between!the!atoms!is!not!symmetrical,!and!atoms!have! a!partial!negative!or!positive ...
Assignment Chemistry Class XI (2016-17)
... 1. Express decimal equivalent of 2/3 to three significance places. 2. The human body temperature is 98.6 0 F. What is value in 0C and K? 3. One atom of an element weights is 9.75 ×10-23 g. calculate its atomic mass. 4.Round up the following to three significant figure (i) 10.4207 (ii) 0.04597 (iii) ...
... 1. Express decimal equivalent of 2/3 to three significance places. 2. The human body temperature is 98.6 0 F. What is value in 0C and K? 3. One atom of an element weights is 9.75 ×10-23 g. calculate its atomic mass. 4.Round up the following to three significant figure (i) 10.4207 (ii) 0.04597 (iii) ...
Lone pairs
... symbol represents the nucleus and the core electrons that do not participate in the chemical bonding. 2) Dots () represent the electrons in the valence energy level of the atom. Arrange these dots around the atomic symbol. ...
... symbol represents the nucleus and the core electrons that do not participate in the chemical bonding. 2) Dots () represent the electrons in the valence energy level of the atom. Arrange these dots around the atomic symbol. ...
1. I can define valence electron and use the periodic
... #4. I can define ion and use the periodic table to predict the ion that is likely to form. 10. What is an ion? 11. What are the two types of ions? 12. Which side of the periodic table are cations on? Why? 13. Which elements will never form ions? 14. An uneven number of _________ and _________ causes ...
... #4. I can define ion and use the periodic table to predict the ion that is likely to form. 10. What is an ion? 11. What are the two types of ions? 12. Which side of the periodic table are cations on? Why? 13. Which elements will never form ions? 14. An uneven number of _________ and _________ causes ...
Biochemistry I (CHE 418 / 5418)
... – Atoms gain or loss or share valence electrons in order to end up with 8 valence electrons. – 8 electrons in the valence shell is a stable electron arrangement. • Helps to explain ions and covalent bonds. – Nonmetals gain electrons. – Metals loss electrons. ...
... – Atoms gain or loss or share valence electrons in order to end up with 8 valence electrons. – 8 electrons in the valence shell is a stable electron arrangement. • Helps to explain ions and covalent bonds. – Nonmetals gain electrons. – Metals loss electrons. ...
Atomic Structure. Chemical Bonds.
... n the principal quantum number l the orbital quantum number ml the magnetic quantum number The fourth quantum number is ms spin magnetic quantum number. ...
... n the principal quantum number l the orbital quantum number ml the magnetic quantum number The fourth quantum number is ms spin magnetic quantum number. ...
2 Types of Chemical Bonds
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
chapter 4-The Structure of Atoms
... performed in the late 1800’s & early 1900’s. –Consist of two electrodes 電極 sealed in a glass tube containing a gas at very low pressure. –When a voltage is applied to the cathodes a glow ...
... performed in the late 1800’s & early 1900’s. –Consist of two electrodes 電極 sealed in a glass tube containing a gas at very low pressure. –When a voltage is applied to the cathodes a glow ...
Introduction to Computational Chemistry
... • Bond lengths and angles of "normal" organic molecules quite accurate (within 2%) • Conformational energies accurate to 1–2 kcal/mol. • Vibrational frequencies for most covalent bonds systematically too high by 10–12% • Zero point vibrational energies: ~1-2 kcal/mol • Isodesmic reaction energies ac ...
... • Bond lengths and angles of "normal" organic molecules quite accurate (within 2%) • Conformational energies accurate to 1–2 kcal/mol. • Vibrational frequencies for most covalent bonds systematically too high by 10–12% • Zero point vibrational energies: ~1-2 kcal/mol • Isodesmic reaction energies ac ...
FXM Rev 1 Key - Grande Cache Community High School
... an element. This number is not found on the periodic table. organic chemistry This is the chemistry of carbon. It is the study of most carbon based compounds. endothermic reaction This is a chemical reaction that requires heat energy to be absorbed to take place. Photosynthesis is this type of react ...
... an element. This number is not found on the periodic table. organic chemistry This is the chemistry of carbon. It is the study of most carbon based compounds. endothermic reaction This is a chemical reaction that requires heat energy to be absorbed to take place. Photosynthesis is this type of react ...
Nuclear Spin - Home - KSU Faculty Member websites
... Let’s start building more complicated atoms to study the Periodic Table. For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) what energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot ...
... Let’s start building more complicated atoms to study the Periodic Table. For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) what energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot ...
1411-Practice Exam 3 (ch6-8)
... B) Na, K, Rb, Cs C) B, Si, As, Te D) F, Cl, Br, I E) Na, Mg, Al, Si ...
... B) Na, K, Rb, Cs C) B, Si, As, Te D) F, Cl, Br, I E) Na, Mg, Al, Si ...
Chapter 8 - TeacherWeb
... The compound has different properties from the elements that make up the compound Ex- NaCl- common table salt ...
... The compound has different properties from the elements that make up the compound Ex- NaCl- common table salt ...
Bio_130_files/Chemistry Review
... – Cations have lost one or more electrons giving them a positive charge(+) • Typically occur between elements on opposite sides of the periodic table. ...
... – Cations have lost one or more electrons giving them a positive charge(+) • Typically occur between elements on opposite sides of the periodic table. ...
Bio_130_files/Chemistry Review
... – Cations have lost one or more electrons giving them a positive charge(+) • Typically occur between elements on opposite sides of the periodic table. ...
... – Cations have lost one or more electrons giving them a positive charge(+) • Typically occur between elements on opposite sides of the periodic table. ...
Click here to Ch 06.2 Covalent Bonding_Lewis Structures
... electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons. ...
... electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons. ...
key
... b) Place the elements in order of increasing ionization energy. K < Li < C < N 109) Which group of the periodic table has elements with high first ionization potentials and very negative electron affinities? Explain this behavior. The halogens. For a given row they have among the highest effective n ...
... b) Place the elements in order of increasing ionization energy. K < Li < C < N 109) Which group of the periodic table has elements with high first ionization potentials and very negative electron affinities? Explain this behavior. The halogens. For a given row they have among the highest effective n ...
Ch 08 MolecularGeometry_rev
... apart as possible – we call this valence shell electron pair repulsion theory – because electrons are negatively charged, they should be most stable when they are separated as much as possible ...
... apart as possible – we call this valence shell electron pair repulsion theory – because electrons are negatively charged, they should be most stable when they are separated as much as possible ...
Nature of Molecules and Water
... – Na atom loses an electron to become Na+ – Cl atom gains an electron to become Cl– – Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound • Electrical attraction of water molecules can disrupt forces holding ions together ...
... – Na atom loses an electron to become Na+ – Cl atom gains an electron to become Cl– – Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound • Electrical attraction of water molecules can disrupt forces holding ions together ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.