Quantum Chemistry Predicts Multiply Bonded Diuranium
... both the staggered and the eclipsed conformation. Analogous to Re2Cl28 , the eclipsed conformation is lower in energy. We have thus optimized the structure for U2Cl28 using an active space formed by 6 active electrons in 13 active orbitals, assuming D4h symmetry. As in the U2Cl6 case, the molecular ...
... both the staggered and the eclipsed conformation. Analogous to Re2Cl28 , the eclipsed conformation is lower in energy. We have thus optimized the structure for U2Cl28 using an active space formed by 6 active electrons in 13 active orbitals, assuming D4h symmetry. As in the U2Cl6 case, the molecular ...
Objective 4
... series of Chemical Reactions in which Sugars are broken down to Carbon Dioxide and Water In this process, energy is released for use by the body. (Breaking Chemical Bonds Releases ...
... series of Chemical Reactions in which Sugars are broken down to Carbon Dioxide and Water In this process, energy is released for use by the body. (Breaking Chemical Bonds Releases ...
EDM Searches Based on Alkali or Alkaline
... configurations. This is the starting configuration for the multireference configuration-interaction 共MRCI兲 关20,21兴 calculation performed in each molecular symmetry group. We use an active space of 兵6,3,3,0其 with no closed orbitals. We used the Stuttgart basis sets and effective core potentials 共ECPs ...
... configurations. This is the starting configuration for the multireference configuration-interaction 共MRCI兲 关20,21兴 calculation performed in each molecular symmetry group. We use an active space of 兵6,3,3,0其 with no closed orbitals. We used the Stuttgart basis sets and effective core potentials 共ECPs ...
Two valence electrons.
... they are almost completely inactive. All are colorless gases. Argon is the most abundant, making up almost one percent of the atmosphere. ...
... they are almost completely inactive. All are colorless gases. Argon is the most abundant, making up almost one percent of the atmosphere. ...
effective nuclear charge
... nucleus and repelled by each other outer electrons are shielded from full strength of nucleus ◦ screening effect effective nuclear charge is net positive charge that is attracting a particular electron Z is nuclear charge, S is electrons in lower energy levels ◦ electrons in same energy level contri ...
... nucleus and repelled by each other outer electrons are shielded from full strength of nucleus ◦ screening effect effective nuclear charge is net positive charge that is attracting a particular electron Z is nuclear charge, S is electrons in lower energy levels ◦ electrons in same energy level contri ...
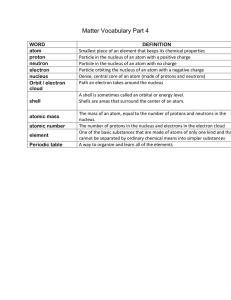
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
Electron Arrangement
... bonding): Hydrogen (H2), Oxygen (O2), Nitrogen (N2) and all of the Group 7 elements (Halogens). In molecular compounds When non-metal atoms join! Eg. Water (H2O), ammonia (NH3), Methane (CH4). These have specific shapes because of the covalent bonds. Covalent molecular substances tend to have low me ...
... bonding): Hydrogen (H2), Oxygen (O2), Nitrogen (N2) and all of the Group 7 elements (Halogens). In molecular compounds When non-metal atoms join! Eg. Water (H2O), ammonia (NH3), Methane (CH4). These have specific shapes because of the covalent bonds. Covalent molecular substances tend to have low me ...
2016 update to LO
... Now that we understand the basics of the solid state, we can turn our attention to more complex solids. Some solids are made of main group elements (the s and p blocks), while other also contain transition metals (the d block). The colors of transition metal compounds are highly variable. Aqueous so ...
... Now that we understand the basics of the solid state, we can turn our attention to more complex solids. Some solids are made of main group elements (the s and p blocks), while other also contain transition metals (the d block). The colors of transition metal compounds are highly variable. Aqueous so ...
Chem. 121, Sec 11 Name: Student I.D. Please Show Your Work
... 8. Determine the relative rates of diffusion of hydrogen gas and oxygen gas at 25◦C? (3 marks) ...
... 8. Determine the relative rates of diffusion of hydrogen gas and oxygen gas at 25◦C? (3 marks) ...
vsepr_lite_oct_2011 - chemistry11crescentsummer
... understand covalent bonding—polar and non-polar be able to draw Lewis structures for simple molecules and polyatomic ions, including molecules with double and triple bonds Introduction The premise of VSEPR theory: In a molecule or polyatomic ion, pairs of valence electrons on the central atom (c ...
... understand covalent bonding—polar and non-polar be able to draw Lewis structures for simple molecules and polyatomic ions, including molecules with double and triple bonds Introduction The premise of VSEPR theory: In a molecule or polyatomic ion, pairs of valence electrons on the central atom (c ...
Chemistry Comes Alive: Part A
... • Heterogeneous translucent mixtures, e.g., cytosol • Large solute particles that do not settle out • Undergo sol-gel transformations ...
... • Heterogeneous translucent mixtures, e.g., cytosol • Large solute particles that do not settle out • Undergo sol-gel transformations ...
Atom (A) or Ion
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Atom (A) or Ion (I)
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Worksheet 20.2
... 1- Atoms can achieve a noble gas structure by gaining, losing or sharing electrons with other atoms. 2- The rule states that, except for hydrogen , an atom combines with other atoms to form bonds in order to have 8 electrons in its valence energy level ( like noble gases). Lewis dot symbols are repr ...
... 1- Atoms can achieve a noble gas structure by gaining, losing or sharing electrons with other atoms. 2- The rule states that, except for hydrogen , an atom combines with other atoms to form bonds in order to have 8 electrons in its valence energy level ( like noble gases). Lewis dot symbols are repr ...
Units 3 and 4 Revision
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
... iron and carbon dioxide. This reaction is shown by the following equation which is not balanced. Fe2 O3 + CO Fe + CO2 Rewrite this as a balanced equation. Fe2 O3 + Standard Grade Chemistry ...
Midterm Review Date
... shared with nitrogen. B) Nitrogen provides a pair of electrons to be shared with hydrogen. C) Hydrogen transfers a pair of electrons to nitrogen. D) Nitrogen transfers a pair of electrons to ...
... shared with nitrogen. B) Nitrogen provides a pair of electrons to be shared with hydrogen. C) Hydrogen transfers a pair of electrons to nitrogen. D) Nitrogen transfers a pair of electrons to ...
The chemical building blocks of life Carbon
... tend to reduce the superposition of p orbitals The properties of silicon and carbon are quite different in many respects - For instance, the electronegativities (i.e. the tendency to attract electrons) are different - According to the Pauling’s scale of electronegativity: !(H)=2.2 !(C)=2.55 ...
... tend to reduce the superposition of p orbitals The properties of silicon and carbon are quite different in many respects - For instance, the electronegativities (i.e. the tendency to attract electrons) are different - According to the Pauling’s scale of electronegativity: !(H)=2.2 !(C)=2.55 ...
File
... 24. What are Valence electrons? Electrons on the outermost energy level of an atom 25. How many electrons can the 1st, 2nd and 3rd energy levels hold? 1st level can hold up to 2; 2nd level can hold up to 8; 3rd level can up hold up to 8 (with exceptions with periods past the 3rd level) 26. Which gro ...
... 24. What are Valence electrons? Electrons on the outermost energy level of an atom 25. How many electrons can the 1st, 2nd and 3rd energy levels hold? 1st level can hold up to 2; 2nd level can hold up to 8; 3rd level can up hold up to 8 (with exceptions with periods past the 3rd level) 26. Which gro ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.