Spillover in Heterogeneous Catalysis - ACS Publications
... where it reacts. The lower figure represents the removal of hydroxyls (as HzO) from a reducible metal oxide to expose underlying metal atoms on the right, and the removal of surface coke (as CH3 from the support on the ...
... where it reacts. The lower figure represents the removal of hydroxyls (as HzO) from a reducible metal oxide to expose underlying metal atoms on the right, and the removal of surface coke (as CH3 from the support on the ...
free sample
... 20) Choose the statement below that is TRUE. A) A weak acid solution consists of mostly nonionized acid molecules. B) The term "strong electrolyte" means that the substance is extremely reactive. C) A strong acid solution consists of only partially ionized acid molecules. D) The term "weak electroly ...
... 20) Choose the statement below that is TRUE. A) A weak acid solution consists of mostly nonionized acid molecules. B) The term "strong electrolyte" means that the substance is extremely reactive. C) A strong acid solution consists of only partially ionized acid molecules. D) The term "weak electroly ...
CHAPTER 9 Stoichiometry - Modern Chemistry Textbook
... chemists also began to experiment with air, which was generally believed to be an element. In 1772, when Daniel Rutherford found that a mouse kept in a closed container soon died, he explained the results based on the phlogiston theory. Like a burning candle, the mouse emitted phlogiston; when the a ...
... chemists also began to experiment with air, which was generally believed to be an element. In 1772, when Daniel Rutherford found that a mouse kept in a closed container soon died, he explained the results based on the phlogiston theory. Like a burning candle, the mouse emitted phlogiston; when the a ...
Cookies and Chemistry…Huh!?!?
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
Chapter+12
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
... What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we ...
2 - cloudfront.net
... Sodium chloride is decomposed into the elements sodium and chlorine gas by means of electrical energy. How much chlorine gas, in grams, is obtained when you have 2.50 mol NaCl? ...
... Sodium chloride is decomposed into the elements sodium and chlorine gas by means of electrical energy. How much chlorine gas, in grams, is obtained when you have 2.50 mol NaCl? ...
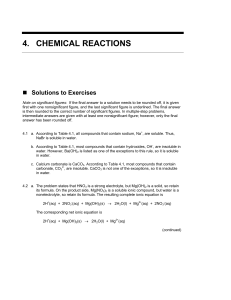
4. chemical reactions
... C2H3O2–, and CN–. Examples of A for reaction c. include S2–, CO32–, and C4H4O62–. 4.4 a. In order to answer this question, you need to compare the number of atoms of X per unit of volume. In order to compare volumes, use the lines on the sides of the beakers. Beaker A has concentration of five atoms ...
... C2H3O2–, and CN–. Examples of A for reaction c. include S2–, CO32–, and C4H4O62–. 4.4 a. In order to answer this question, you need to compare the number of atoms of X per unit of volume. In order to compare volumes, use the lines on the sides of the beakers. Beaker A has concentration of five atoms ...
Synthesis, Characterization and Properties of Copper Clusters
... confinement effect. The main purpose of this thesis has been the synthesis of copper clusters as an affordable alternative to the most studied clusters up to now: gold and silver clusters. In this dissertation, firstly, we have explored the synthesis of different copper clusters (CuCLs) sizes and th ...
... confinement effect. The main purpose of this thesis has been the synthesis of copper clusters as an affordable alternative to the most studied clusters up to now: gold and silver clusters. In this dissertation, firstly, we have explored the synthesis of different copper clusters (CuCLs) sizes and th ...
Chemistry Appendixes
... questions by applying consistent, logical reasoning to describe, explain, and predict observations, and by doing experiments to test hypotheses or predictions from these hypotheses. In this way science progresses using a general model for solving problems and employing specific processes as part of ...
... questions by applying consistent, logical reasoning to describe, explain, and predict observations, and by doing experiments to test hypotheses or predictions from these hypotheses. In this way science progresses using a general model for solving problems and employing specific processes as part of ...
Schaum`s Outline of Theory and Problems of
... you use a different symbol, you might become confused later when that symbol is used for a different quantity. Some of the problems are stated in parts. After you do the problem by solving the various parts, see if you would know how to solve the same problem if only the last part were asked. The co ...
... you use a different symbol, you might become confused later when that symbol is used for a different quantity. Some of the problems are stated in parts. After you do the problem by solving the various parts, see if you would know how to solve the same problem if only the last part were asked. The co ...
CHAPTER 9
... After obtaining similar results by using various substances, Lavoisier concluded that air was not an element but a mixture composed principally of two gases, Priestley’s “dephlogisticated air” (which Lavoisier renamed oxygen) and Rutherford’s “phlogisticated air” (which was mostly nitrogen but had t ...
... After obtaining similar results by using various substances, Lavoisier concluded that air was not an element but a mixture composed principally of two gases, Priestley’s “dephlogisticated air” (which Lavoisier renamed oxygen) and Rutherford’s “phlogisticated air” (which was mostly nitrogen but had t ...
Corrosion of Aluminium and Zinc-Aluminium Alloys Based Metal
... corrosion. Pitting corrosion occurs when discrete areas of a material undergo rapid attack while most of the adjacent surface remains virtually unaffected. Apart from the localized loss of thickness, corrosion pits can also be harmful by acting as stress concentrators. Stress corrosion cracking and ...
... corrosion. Pitting corrosion occurs when discrete areas of a material undergo rapid attack while most of the adjacent surface remains virtually unaffected. Apart from the localized loss of thickness, corrosion pits can also be harmful by acting as stress concentrators. Stress corrosion cracking and ...
CHAPTER 9 Notes
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
Laboratory Works and Home Tasks in General Chemistry
... Having calculated the normality of the analyzed solution using the titration results we can determine the mass of the substance in any volume of the solution: m(X) = CN(X) ⋅ M(X) · feq(X) · V. To conduct successfully the titration analysis you should: 1) know the exact concentration of the titrant ( ...
... Having calculated the normality of the analyzed solution using the titration results we can determine the mass of the substance in any volume of the solution: m(X) = CN(X) ⋅ M(X) · feq(X) · V. To conduct successfully the titration analysis you should: 1) know the exact concentration of the titrant ( ...
File
... present (at constant T and P). The magnitude of G equals wmax. When G < 0, the magnitude tells us how much work, in theory, could be harnessed from the reaction. When G > 0, the magnitude tells us the minimum amount of work that must be supplied to make the reaction occur. G gives us the same i ...
... present (at constant T and P). The magnitude of G equals wmax. When G < 0, the magnitude tells us how much work, in theory, could be harnessed from the reaction. When G > 0, the magnitude tells us the minimum amount of work that must be supplied to make the reaction occur. G gives us the same i ...
Go FIGure
... nearly nonpolar, and the molecules are symmetrical enough to cancel much of the weak C ¬ H bond dipoles. The attraction between the polar water molecules and the nonpolar hydrocarbon molecules is not sufficiently strong to allow the formation of a solution. Nonpolar liquids tend to be insoluble in p ...
... nearly nonpolar, and the molecules are symmetrical enough to cancel much of the weak C ¬ H bond dipoles. The attraction between the polar water molecules and the nonpolar hydrocarbon molecules is not sufficiently strong to allow the formation of a solution. Nonpolar liquids tend to be insoluble in p ...
© www.CHEMSHEETS.co.uk 17-Jul
... it is best to show each separate step (e.g. if both elements are atomised, show this as two steps) ...
... it is best to show each separate step (e.g. if both elements are atomised, show this as two steps) ...
X Science Practice Paper - Brilliant Public School Sitamarhi
... Q 19 Why does distilled water not conduct electricity, whereas rainwater does? Marks (2) Q 20 Fresh milk has a pH 6. How do you think the pH will change as it becomes sour? Marks (2) Q 21 What are acid & base indicators? Marks (2) Q 22 What are alkalis? Marks (2) Q 23 Why does an aqueous solution of ...
... Q 19 Why does distilled water not conduct electricity, whereas rainwater does? Marks (2) Q 20 Fresh milk has a pH 6. How do you think the pH will change as it becomes sour? Marks (2) Q 21 What are acid & base indicators? Marks (2) Q 22 What are alkalis? Marks (2) Q 23 Why does an aqueous solution of ...
Laboratories to be performed
... your minutes when I report them to the Dean’s office. Message: Don’t be late! 2. You should keep all notes, handouts, worksheets, homework assignments, tests, and quizzes together in one location, preferably in a 3-ring binder that should be specific for this class. Since this is an AP course, the e ...
... your minutes when I report them to the Dean’s office. Message: Don’t be late! 2. You should keep all notes, handouts, worksheets, homework assignments, tests, and quizzes together in one location, preferably in a 3-ring binder that should be specific for this class. Since this is an AP course, the e ...
Workshop materials for Class XII
... Consider this from your perspective, and from the point of view of the people around you. Don’t be modest or shy , be as objective as you can. If you have any difficulty with this write down a list of your personal charecteristics. Some of these will hopefully be strengths. ...
... Consider this from your perspective, and from the point of view of the people around you. Don’t be modest or shy , be as objective as you can. If you have any difficulty with this write down a list of your personal charecteristics. Some of these will hopefully be strengths. ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.