Anexo I. Modelos teóricos de análisis de sistemas de valencia mixta.
... transferencias de carga en compuestos de valencia mixta organometálicos 7 , 8 y orgánicos 9,10 , así como también en transferencias de carga ligando-metal o metalligando. 11, 12 La validez del modelo de Hush (publicado a finales de los años 60)5 se limita a sistemas de valencia mixta poco acoplados ...
... transferencias de carga en compuestos de valencia mixta organometálicos 7 , 8 y orgánicos 9,10 , así como también en transferencias de carga ligando-metal o metalligando. 11, 12 La validez del modelo de Hush (publicado a finales de los años 60)5 se limita a sistemas de valencia mixta poco acoplados ...
SCH3U: Final Exam Review
... Calculate the volume that is occupied by 5.05 mol of hydrogen chloride, HCl, gas at STP. What is the pressure of 6.7 mol of carbon dioxide gas, in 35.0 L at 30°C? Calculate the volume of water vapour that is produced from the combustion of 15.0 g of ethylene at 25°C and 100 kPa. C2H4(g) + 3O2(g) → 2 ...
... Calculate the volume that is occupied by 5.05 mol of hydrogen chloride, HCl, gas at STP. What is the pressure of 6.7 mol of carbon dioxide gas, in 35.0 L at 30°C? Calculate the volume of water vapour that is produced from the combustion of 15.0 g of ethylene at 25°C and 100 kPa. C2H4(g) + 3O2(g) → 2 ...
2. Solution Guide to Supplementary Exercises
... Option B — A fuse is a safety device that protects electric circuits from the effects of excessive electric currents. It commonly consists of a current-conducting wire of low melting point. If we try to pass a current higher than the rated value through the wire, it will heat up so much that it melt ...
... Option B — A fuse is a safety device that protects electric circuits from the effects of excessive electric currents. It commonly consists of a current-conducting wire of low melting point. If we try to pass a current higher than the rated value through the wire, it will heat up so much that it melt ...
- Boreskov Institute of Catalysis
... More than the 50-year-long history of the Institute is a glowing example of fruitful interaction between fundamental science and industry and of the profound realization that solving scientific problems should be aimed at some practical result. The accomplishments of the Institute embody its origina ...
... More than the 50-year-long history of the Institute is a glowing example of fruitful interaction between fundamental science and industry and of the profound realization that solving scientific problems should be aimed at some practical result. The accomplishments of the Institute embody its origina ...
- Chemistry
... The standard molar enthalpy of formation of liquid methanol, CH3OH(l), is the standard enthalpy change of the following reaction: ...
... The standard molar enthalpy of formation of liquid methanol, CH3OH(l), is the standard enthalpy change of the following reaction: ...
b - Gordon State College
... The titration of a 10.00-mL sample of an HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? ...
... The titration of a 10.00-mL sample of an HCl solution of unknown concentration requires 12.54 mL of a 0.100 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? ...
Part 3-ICHO-31-35
... and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in a bomb calorimeter, which initially contains 710.0 g of water at 25.000 °C. After the r ...
... and –285.83 kJ mol-1, respectively. The gas constant, R = 8.314 J K-1 mol-1. (Relative atomic masses : H = 1.0; C = 12.0; O = 16.0) A sample of solid Q that weighs 0.6000 g, is combusted in an excess of oxygen in a bomb calorimeter, which initially contains 710.0 g of water at 25.000 °C. After the r ...
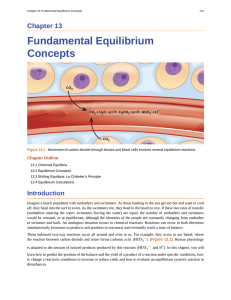
Fundamental Equilibrium Concepts
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
... Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are ...
Mastering the Chemistry Core 40
... For each chapter in the Glencoe textbook, Chemistry: Matter and Change, two pages of chapter review questions have been provided. These questions are designed to test your comprehension of chapter content and provide you with practice for your test-taking skills. All of the questions are in a multip ...
... For each chapter in the Glencoe textbook, Chemistry: Matter and Change, two pages of chapter review questions have been provided. These questions are designed to test your comprehension of chapter content and provide you with practice for your test-taking skills. All of the questions are in a multip ...
Chapter 4 - Chemistry
... The oxidation number for hydrogen is 1 (rule 4), and for oxygen is 2 (rule 3). The oxidation number for sulfur in S8 is zero (rule 1). Remember that in a neutral molecule, the sum of the oxidation numbers of all the atoms must be zero, and in an ion the sum of oxidation numbers of all elements in ...
... The oxidation number for hydrogen is 1 (rule 4), and for oxygen is 2 (rule 3). The oxidation number for sulfur in S8 is zero (rule 1). Remember that in a neutral molecule, the sum of the oxidation numbers of all the atoms must be zero, and in an ion the sum of oxidation numbers of all elements in ...
Question Bank (Class XI - Chemistry)
... The formulae for all reactants and products must be correct. It should be arithmetically balanced, i.e., the number of atoms of each element on both sides of arrow should be equal. ...
... The formulae for all reactants and products must be correct. It should be arithmetically balanced, i.e., the number of atoms of each element on both sides of arrow should be equal. ...
4134gdisk doc..4134gdisk chapter .. Page501
... the reaction of [tris(pyrazoyl)hydroborato]zinc hydroxide has been probed using both theoretical and experimental methods. Removal of Cu from the enzyme from Hansenula polymorha has the effect of discontinuing enzyme activity.21 The enzyme was active, however, when Co2+ was intoduced and it is concl ...
... the reaction of [tris(pyrazoyl)hydroborato]zinc hydroxide has been probed using both theoretical and experimental methods. Removal of Cu from the enzyme from Hansenula polymorha has the effect of discontinuing enzyme activity.21 The enzyme was active, however, when Co2+ was intoduced and it is concl ...
Chapter 2 The Electroless Nickel Plating Bath: Effect of Variables on
... no anodes available to maintain a near-constant metal concentration, and no external electron source (rectifier) to keep a constant flow of electrons moving into the system, as in an electrolytic plating process. In order to have a continuous and consistent electroless plating process, the reactants ...
... no anodes available to maintain a near-constant metal concentration, and no external electron source (rectifier) to keep a constant flow of electrons moving into the system, as in an electrolytic plating process. In order to have a continuous and consistent electroless plating process, the reactants ...
10. Solution Guide to Supplementary Exercises
... In experiment A, 1.00 mol dm–3 H2(g) and 1.00 mol dm–3 I2(g) were initially added to a flask and equilibrium was established. In experiment B, 2.00 mol dm–3 HI(g) were initially added to a second flask and equilibrium was established. Which of the following statements is always true about the equili ...
... In experiment A, 1.00 mol dm–3 H2(g) and 1.00 mol dm–3 I2(g) were initially added to a flask and equilibrium was established. In experiment B, 2.00 mol dm–3 HI(g) were initially added to a second flask and equilibrium was established. Which of the following statements is always true about the equili ...
Section 1
... Atoms are the particles whose symbols are found in the periodic table of elements given in all your examination papers and also in Section 12 of this workbook. You can see that there are only about 100 of them. The middle part of the atom, the nucleus, contains one or more protons. It is the number ...
... Atoms are the particles whose symbols are found in the periodic table of elements given in all your examination papers and also in Section 12 of this workbook. You can see that there are only about 100 of them. The middle part of the atom, the nucleus, contains one or more protons. It is the number ...
The science of chemistry is concerned
... 2.4 and 2.6, in which the Avogadro constant and molar mass were used as conversion factors. As in these previous cases, there is no need to memorize or do algebraic manipulations with Eq. (3.3) when using the stoichiometric ratio. Simply remember that the coefficients in a balanced chemical equation ...
... 2.4 and 2.6, in which the Avogadro constant and molar mass were used as conversion factors. As in these previous cases, there is no need to memorize or do algebraic manipulations with Eq. (3.3) when using the stoichiometric ratio. Simply remember that the coefficients in a balanced chemical equation ...
Decrease = stress More Fe(OH) 2 dissolves in response Solubility
... • Ksp used to compare relative solubilities –smaller Ksp = less soluble –larger Ksp= more soluble ...
... • Ksp used to compare relative solubilities –smaller Ksp = less soluble –larger Ksp= more soluble ...
UNIT 1. SOME BASIC CONCEPTS OF CHEMISTRY Concept
... The formulae for all reactants and products must be correct. It should be arithmetically balanced, i.e., the number of atoms of each element on both sides of arrow should be equal. ...
... The formulae for all reactants and products must be correct. It should be arithmetically balanced, i.e., the number of atoms of each element on both sides of arrow should be equal. ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.