chemistry advanced may 2010 marking scheme
... e.g. ammonium chloride + NaOH(aq); soda lime + CH3COONa or other suitable balanced chemical equations (1, 1) Note: accept reaction of Be2C with acid or alkali for methane: e.g. Be2C + 4NaOH → 2Na2BeO2 + CH4; with water reaction is very slow (award only 0.5) 5. This question is about the chemistry of ...
... e.g. ammonium chloride + NaOH(aq); soda lime + CH3COONa or other suitable balanced chemical equations (1, 1) Note: accept reaction of Be2C with acid or alkali for methane: e.g. Be2C + 4NaOH → 2Na2BeO2 + CH4; with water reaction is very slow (award only 0.5) 5. This question is about the chemistry of ...
Chemistry Answers - Heathcote School and Science College
... Melting and boiling Low – weak forces High – strong points (with between molecules attraction between reason) positive and negative ions ...
... Melting and boiling Low – weak forces High – strong points (with between molecules attraction between reason) positive and negative ions ...
CHEM 400 - El Camino College
... , where m is the mass of one molecule of gas. At the same temperature larger ...
... , where m is the mass of one molecule of gas. At the same temperature larger ...
Normality Primer
... To know how many electrons are gained or lost, redox reactions require knowledge of the half reaction associated with the reducing or oxidizing agent. These reactions are not easily predictable and generally chemists look them up in tables of reduction potentials. However, one must be particula ...
... To know how many electrons are gained or lost, redox reactions require knowledge of the half reaction associated with the reducing or oxidizing agent. These reactions are not easily predictable and generally chemists look them up in tables of reduction potentials. However, one must be particula ...
Honors Chemistry Chapter 14 notes—Acids, Bases, and pH I. Acids
... b. therefore acids generally speaking corrode most metals c. generally speaking this corrosion is a single-displacement reaction (remember Chapter 6?) d. acids will also react with carbonates (ex. CaCO3) to release CO2, water, and another compound e. Marble and Limestone sculptures are affected by a ...
... b. therefore acids generally speaking corrode most metals c. generally speaking this corrosion is a single-displacement reaction (remember Chapter 6?) d. acids will also react with carbonates (ex. CaCO3) to release CO2, water, and another compound e. Marble and Limestone sculptures are affected by a ...
Final Exam Review
... fundamental unit of measurement is multiplied by what factor? a. 10-3 b. 10-2 c. 10-6 d. 103 e. 106 11. The measurement most likely to describe the amount of pain reliever in a headache tablet is a. 1.5 kg b. 500 mg c. 1.00 mL d. 325 mg/mL e. 0.25 L 12. Which of the following measurements has three ...
... fundamental unit of measurement is multiplied by what factor? a. 10-3 b. 10-2 c. 10-6 d. 103 e. 106 11. The measurement most likely to describe the amount of pain reliever in a headache tablet is a. 1.5 kg b. 500 mg c. 1.00 mL d. 325 mg/mL e. 0.25 L 12. Which of the following measurements has three ...
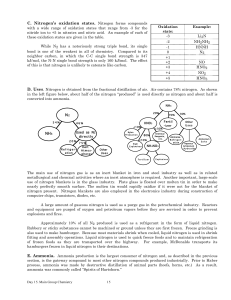
Nitrogen`s oxidation states
... electronegativities of the elements increase. Oxygen's electronegativity (3.5) is quite large and second only to fluorine's. All of the non-metals have electronegativities that are greater than 2.0 so the difference in electronegativities between a non-metal and oxygen is always less than 1.5. Elect ...
... electronegativities of the elements increase. Oxygen's electronegativity (3.5) is quite large and second only to fluorine's. All of the non-metals have electronegativities that are greater than 2.0 so the difference in electronegativities between a non-metal and oxygen is always less than 1.5. Elect ...
Chemistry II Exams and Answer Keys 2015 Season
... 16. Scuba stands for self-contained underwater breathing apparatus. A scuba tank is a gas cylinder used to store and transport high pressure gases for scuba divers. When high pressure gases in the scuba tank come in contact with water in the blood stream, these gases dissolve into the blood stream. ...
... 16. Scuba stands for self-contained underwater breathing apparatus. A scuba tank is a gas cylinder used to store and transport high pressure gases for scuba divers. When high pressure gases in the scuba tank come in contact with water in the blood stream, these gases dissolve into the blood stream. ...
3 CO 2(g)
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
The masses of reactants and products are equal.
... equation, so C is balanced. However, on the left side, H has a subscript of 4, which means there are four hydrogen atoms. On the right side, H has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen atoms on the left and three oxygen atoms on the right. Because of ...
... equation, so C is balanced. However, on the left side, H has a subscript of 4, which means there are four hydrogen atoms. On the right side, H has a subscript of 2, which means there are two hydrogen atoms. Also, there are two oxygen atoms on the left and three oxygen atoms on the right. Because of ...
Chemistry Final Exam Study Guide
... c. scientific law b. theory d. observation ____ 26. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 27. Which of the following would a chemist be most likely to study? a. a leaf floating on water c. a leaf being blown by the ...
... c. scientific law b. theory d. observation ____ 26. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 27. Which of the following would a chemist be most likely to study? a. a leaf floating on water c. a leaf being blown by the ...
Chapter 5HW_Ans
... b) conversion factor: x X moles of C2H2; therefore 7 moles of O2 2molesC 2 H 2 as moles of C2H2 cancel as they are in both numerator and denominator 2molesC 2 H 2 c) conversion factor: x X moles of H2O; therefore 0.5 moles of 2molesH 2 O C2H2 as moles of H2O cancel as they are in both numerator and ...
... b) conversion factor: x X moles of C2H2; therefore 7 moles of O2 2molesC 2 H 2 as moles of C2H2 cancel as they are in both numerator and denominator 2molesC 2 H 2 c) conversion factor: x X moles of H2O; therefore 0.5 moles of 2molesH 2 O C2H2 as moles of H2O cancel as they are in both numerator and ...
Energy of Reactions
... You need to have known chemical reactions that you can combine to form the final chemical reaction You need the δH for each reaction If you need to reverse a reaction, you must also change the sign of δH If you need to multiply a reaction by a number, you must also multiply the δH When all reactions ...
... You need to have known chemical reactions that you can combine to form the final chemical reaction You need the δH for each reaction If you need to reverse a reaction, you must also change the sign of δH If you need to multiply a reaction by a number, you must also multiply the δH When all reactions ...
Test
... a. They describe the phase the substance is in. b. They describe characteristics of a substance such as size, color, and shape. c. They explain how the substance reacts with other things d. They describe what chemical changes the substance is currently going through. While investigating a new substa ...
... a. They describe the phase the substance is in. b. They describe characteristics of a substance such as size, color, and shape. c. They explain how the substance reacts with other things d. They describe what chemical changes the substance is currently going through. While investigating a new substa ...
Naming Ionic Compounds 16 Naming Ionic Compounds
... d. In what region of the periodic table are these “multiple ion” elements usually located? 2. Consider the ions of potassium (K) and sulfur (S). Write chemical formulas for all possible ionic compounds involving these ions, using the simplest ratio(s) of potassium (K) and sulfur (S). Keep in min ...
... d. In what region of the periodic table are these “multiple ion” elements usually located? 2. Consider the ions of potassium (K) and sulfur (S). Write chemical formulas for all possible ionic compounds involving these ions, using the simplest ratio(s) of potassium (K) and sulfur (S). Keep in min ...
AP Chem Summer Assign Gen Chem Rev Problems
... CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) a. If 1.20x1024 molecules of CO2 is exhaled, the average amount exhaled by a person each day, how much (in grams) Li2CO3 is produced? b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to co ...
... CO2(g) + 2LiOH(s) → Li2CO3(s) + H2O(l) a. If 1.20x1024 molecules of CO2 is exhaled, the average amount exhaled by a person each day, how much (in grams) Li2CO3 is produced? b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to co ...
Electricity - Micron Technology, Inc.
... A: Help the students think of materials that transmit electric current. Examples include: gold, copper, aluminum, and other metals. Q: What is the opposite of a conductor? Who can give an example? A: Insulators are the opposite of conductors. Insulators are materials that have tightly bound electron ...
... A: Help the students think of materials that transmit electric current. Examples include: gold, copper, aluminum, and other metals. Q: What is the opposite of a conductor? Who can give an example? A: Insulators are the opposite of conductors. Insulators are materials that have tightly bound electron ...
2009 - NESACS
... 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make Be-8 which is then struck by a third α particle, creating C-12. This improbable sequence is called the triple- ...
... 100 million K deep inside giant red star core where H is all consumed and He is in abundance. Unstable Be-8 is crucial in creating C-12 but for a split second, 2 He−4 particles fuse to make Be-8 which is then struck by a third α particle, creating C-12. This improbable sequence is called the triple- ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.