Name: (1 of 2) Math Set # 13 Protons, Neutrons, Electrons Proton
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
• • • • • • • • • • • • • • • • • • • • • • • • • •
... Define oxidation and reduction in terms of a change in oxidation number and identify atoms being oxidized or reduced in redox reactions. Balancing Redox Reactions Describe how oxidation numbers are used to identify redox reactions. Balance a redox equation using the oxidation-number-change met ...
... Define oxidation and reduction in terms of a change in oxidation number and identify atoms being oxidized or reduced in redox reactions. Balancing Redox Reactions Describe how oxidation numbers are used to identify redox reactions. Balance a redox equation using the oxidation-number-change met ...
g) Chemistry 30 - Mr. Jones LHS Science
... d. If C6H6 (g), were consumed instead of C6H6 (l), would you expect the magnitude of H to increase, decrease, or stay the same? Explain. ...
... d. If C6H6 (g), were consumed instead of C6H6 (l), would you expect the magnitude of H to increase, decrease, or stay the same? Explain. ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... For example: H2O2 HO OR C6H12O6 CH2O (Take the lowest subscript number and divide it into the other subscripts.) VII. Energy and Molecules A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond fo ...
... For example: H2O2 HO OR C6H12O6 CH2O (Take the lowest subscript number and divide it into the other subscripts.) VII. Energy and Molecules A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond fo ...
Summer Work
... when dissolved in water. It is marketed by G.D. Searle as Nutra Sweet. The molecular formula of aspartame is C14H18N2O5 . a) Calculate the gram-formula-mass of aspartame. ...
... when dissolved in water. It is marketed by G.D. Searle as Nutra Sweet. The molecular formula of aspartame is C14H18N2O5 . a) Calculate the gram-formula-mass of aspartame. ...
a level chemistry - some definitions to learn
... The simplest, whole number, ratio of elements in a compound The exact number of atoms of each element in the formula of a compound Oppositely charged ions held together in a crystal lattice by electrostatic attraction A shared pair of electrons, one electron being supplied by each atom either side o ...
... The simplest, whole number, ratio of elements in a compound The exact number of atoms of each element in the formula of a compound Oppositely charged ions held together in a crystal lattice by electrostatic attraction A shared pair of electrons, one electron being supplied by each atom either side o ...
3C95 Chemistry 12 2015-2016 (Lockwood)
... D3 analyse balanced equations representing the reaction of acids and bases with water 1. identify Brønsted-Lowry acids and bases in an equation 2. define conjugate acid-base pair 3. identify the conjugate of a given acid or base 4. show that in any Brønsted-Lowry acid-base equation there are two con ...
... D3 analyse balanced equations representing the reaction of acids and bases with water 1. identify Brønsted-Lowry acids and bases in an equation 2. define conjugate acid-base pair 3. identify the conjugate of a given acid or base 4. show that in any Brønsted-Lowry acid-base equation there are two con ...
4.1 PPT- Atomic Theory and Bonding
... Atoms gain and lose electrons in an attempt to be STABLE. The noble gases are stable because they have FULL outer shells of electrons. They don’t need to lose or gain any e-s. Atoms in each period want to have the same number of electrons in their outer shell (VALENCE ELECTRONS) as the noble gases ...
... Atoms gain and lose electrons in an attempt to be STABLE. The noble gases are stable because they have FULL outer shells of electrons. They don’t need to lose or gain any e-s. Atoms in each period want to have the same number of electrons in their outer shell (VALENCE ELECTRONS) as the noble gases ...
Science 10 student notes
... was found face down in the dining room. Looking into the room, you start our investigation. The large window in the dining room has been shattered and appears to have been smashed open from the outside. The body exhibits laceration wounds and lies face down by the table, and there is a large red sta ...
... was found face down in the dining room. Looking into the room, you start our investigation. The large window in the dining room has been shattered and appears to have been smashed open from the outside. The body exhibits laceration wounds and lies face down by the table, and there is a large red sta ...
Preparation and Characterization of a Dip-Coated SnO2
... A new method for the synthesis of SnO2 is proposed and thin films are prepared by a dip-coating method. In the present paper we report that these SnO2 films exhibit a reversible electrochemical insertion of lithium ions while maintaining high optical transmissivity. These films can be used as transp ...
... A new method for the synthesis of SnO2 is proposed and thin films are prepared by a dip-coating method. In the present paper we report that these SnO2 films exhibit a reversible electrochemical insertion of lithium ions while maintaining high optical transmissivity. These films can be used as transp ...
Lab 3. Chemical Reactions
... rolled into pellets, or pounded into sheets and foil). It conducts heat and electricity but is not magnetic. It has a high melting point (it takes a lot of energy to make it turn from a solid to a liquid) and it is not soluble (doesn’t dissolve) in water. These characteristics are physical propertie ...
... rolled into pellets, or pounded into sheets and foil). It conducts heat and electricity but is not magnetic. It has a high melting point (it takes a lot of energy to make it turn from a solid to a liquid) and it is not soluble (doesn’t dissolve) in water. These characteristics are physical propertie ...
physical setting chemistry
... question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You may use scrap paper to work out the answers to the questions, but be sure to ...
... question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You may use scrap paper to work out the answers to the questions, but be sure to ...
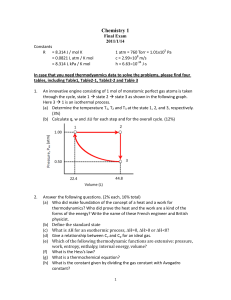
Chemistry 1
... 12. You wish to construct a fuel cell to do electrical work for you based on the oxidation of a hydrocarbon fuel. To understand how it works, you will (a) derive the relation between Gibbs free energy and the maximum non-expansion work at constant T and P (5%), (b) calculate the maximum non-expansio ...
... 12. You wish to construct a fuel cell to do electrical work for you based on the oxidation of a hydrocarbon fuel. To understand how it works, you will (a) derive the relation between Gibbs free energy and the maximum non-expansion work at constant T and P (5%), (b) calculate the maximum non-expansio ...
BSPH 111 - Refresher Chemistry
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
Document
... The standard enthalpy of formation, DHf°, is the enthalpy change for the formation of one mole of the substance from its elements in their reference forms and in their standard states. DHf° for an element in its reference and standard state is zero. For example, the standard enthalpy of formation f ...
... The standard enthalpy of formation, DHf°, is the enthalpy change for the formation of one mole of the substance from its elements in their reference forms and in their standard states. DHf° for an element in its reference and standard state is zero. For example, the standard enthalpy of formation f ...
AP Chemistry Review Preparing for the AP
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
... Give examples and solve calculation problems related to each of the three theories. Sketch a cathode ray tube as demonstrated in class and state how J.J. Thomson’s experiments led to the idea that atoms have positive and negative parts, the negative parts are all the same, and the negative parts ...
Kinetics and Equilibrium Review Page 1
... Kinetics and Equilibrium Review 35. When AgNO3(aq) is mixed with NaCl(aq), a reaction occurs which tends to go to completion and not reach equilibrium because A) a gas is formed B) water is formed C) a weak acid is formed D) a precipitate is formed 36. The vapor pressure of a liquid at a given temp ...
... Kinetics and Equilibrium Review 35. When AgNO3(aq) is mixed with NaCl(aq), a reaction occurs which tends to go to completion and not reach equilibrium because A) a gas is formed B) water is formed C) a weak acid is formed D) a precipitate is formed 36. The vapor pressure of a liquid at a given temp ...
(H) +
... • Generally do not contain C • Usually smaller than organic molecules • Usually dissociate in water, forming ions • Water, oxygen, carbon dioxide, and inorganic salts ...
... • Generally do not contain C • Usually smaller than organic molecules • Usually dissociate in water, forming ions • Water, oxygen, carbon dioxide, and inorganic salts ...
Chemistry Readings
... that an element has. These groups are sometimes labeled with Roman Numerals. Group IA has one valence electron, group IIA has two valence electrons and the number of valence electrons would continue to go up by one. Group O would have eight valence electrons. The metal elements are on the left and i ...
... that an element has. These groups are sometimes labeled with Roman Numerals. Group IA has one valence electron, group IIA has two valence electrons and the number of valence electrons would continue to go up by one. Group O would have eight valence electrons. The metal elements are on the left and i ...
CATION ANALYSIS - webhosting.au.edu
... identity of an unknown sample. Given a totally “ unknown” sample, how does one go about determining what is actually present? This process is called “ qualitative analysis”. Cations are classified into five groups on the basis of their behavior against some reagents by using group reagents; we can d ...
... identity of an unknown sample. Given a totally “ unknown” sample, how does one go about determining what is actually present? This process is called “ qualitative analysis”. Cations are classified into five groups on the basis of their behavior against some reagents by using group reagents; we can d ...
Summary of 5.4
... Identify the carbon skeleton and the functional groups. If benzene and BrCHCHCOOH react in the presence of iron bromide to make an intermediate state how the mass spectrum and the NMR spectrum would change from reactants to intermediate. State how the IR spectrum of the product would change from the ...
... Identify the carbon skeleton and the functional groups. If benzene and BrCHCHCOOH react in the presence of iron bromide to make an intermediate state how the mass spectrum and the NMR spectrum would change from reactants to intermediate. State how the IR spectrum of the product would change from the ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.