Crystal Field Theory, gemstones and color
... e) given the structures of Beryl and Corundum, explain using a physical model why the two materials have such different harndesses. Breakdown of CFT - covalency One of the useful things about crystal field theory is its predictive nature. Once we know a crystal field splitting pattern and what atoms ...
... e) given the structures of Beryl and Corundum, explain using a physical model why the two materials have such different harndesses. Breakdown of CFT - covalency One of the useful things about crystal field theory is its predictive nature. Once we know a crystal field splitting pattern and what atoms ...
Organic Chemical Reactions
... directions is inserted between reactants and products. However, many organic reactions are almost completely shifted towards the products and the residual concentration of the reactants at the end of the reaction is negligible. This happens when the equilibrium constant is very high. For those react ...
... directions is inserted between reactants and products. However, many organic reactions are almost completely shifted towards the products and the residual concentration of the reactants at the end of the reaction is negligible. This happens when the equilibrium constant is very high. For those react ...
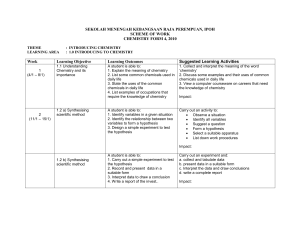
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigate the reactions of chlorine, bromine and iodine with a) water b) metals such as iron c) sodium hydroxide Impact/reflect: Collect and interpret data on the propertie ...
... c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigate the reactions of chlorine, bromine and iodine with a) water b) metals such as iron c) sodium hydroxide Impact/reflect: Collect and interpret data on the propertie ...
rate of chemical reaction and chemical equilibrium
... water and releases chloride ion bound to central platinum metal. The reaction is represented as Pt(NH3)2Cl2 + H2O Pt(NH3)2Cl+ + Cl Here the conc. of cisplatin decreases with lapse of time but conc. of Cl increases. ...
... water and releases chloride ion bound to central platinum metal. The reaction is represented as Pt(NH3)2Cl2 + H2O Pt(NH3)2Cl+ + Cl Here the conc. of cisplatin decreases with lapse of time but conc. of Cl increases. ...
8 SHS Ch 8 Lecture shs_ch_8_lecture_2012
... In a precipitation reaction a product of the reaction is only slightly soluble, or insoluble. This product is formed as a solid, also known as a precipitate. Solubility Rules can be used to determine if a product is insoluble (forms a precipitate) p. 284 ...
... In a precipitation reaction a product of the reaction is only slightly soluble, or insoluble. This product is formed as a solid, also known as a precipitate. Solubility Rules can be used to determine if a product is insoluble (forms a precipitate) p. 284 ...
KS4-Rates - Free Exam Papers
... bonds between atoms often before new given but out as energy bonds can be formed ones have to be new old bonds needed to broken. form break existing • This means that there has to be enough energy bonds (activation energy)to start breaking the old bonds before a reaction can occur. ...
... bonds between atoms often before new given but out as energy bonds can be formed ones have to be new old bonds needed to broken. form break existing • This means that there has to be enough energy bonds (activation energy)to start breaking the old bonds before a reaction can occur. ...
Laboratory Exercises in Physical Chemistry
... a small amount of a solute is called a colligative property. For dilute solutions it depends on the number of the solute particles, but not on any of the properties of the solute particles. These particles could be small molecules, macromolecules or ionic species. Only the number of these particles ...
... a small amount of a solute is called a colligative property. For dilute solutions it depends on the number of the solute particles, but not on any of the properties of the solute particles. These particles could be small molecules, macromolecules or ionic species. Only the number of these particles ...
Electricity - Arlington Public Schools
... the hanging balloon. Students should conclude that balloons can be made to attract and repel each other when they are rubbed with different materials. Students should recognize that electrically charged objects attract or repel each other as can be seen from the effects of static electricity. ...
... the hanging balloon. Students should conclude that balloons can be made to attract and repel each other when they are rubbed with different materials. Students should recognize that electrically charged objects attract or repel each other as can be seen from the effects of static electricity. ...
KISS Notes
... Iron is stronger and harder than bronze. A warrior armed with iron weapons will usually beat a bronzearmed man. Iron tools and even the humble nail allowed new developments in buildings, ships, wagons... remember that towns, trade and commerce give wealth and power. An iron plough allows more land t ...
... Iron is stronger and harder than bronze. A warrior armed with iron weapons will usually beat a bronzearmed man. Iron tools and even the humble nail allowed new developments in buildings, ships, wagons... remember that towns, trade and commerce give wealth and power. An iron plough allows more land t ...
Chapter 1 Introduction - SOIL 4234 Soil Nutrient Management
... • Divalent and trivalent nutrient ions are immobile in soils (exception SO42-) • In tropical soils, are enough anion exchange sites to provide significant adsorption of SO42and cause it to be somewhat immobile. Although sulfate compounds, such as CaSO4 and MgSO4 are relatively insoluble, the equilib ...
... • Divalent and trivalent nutrient ions are immobile in soils (exception SO42-) • In tropical soils, are enough anion exchange sites to provide significant adsorption of SO42and cause it to be somewhat immobile. Although sulfate compounds, such as CaSO4 and MgSO4 are relatively insoluble, the equilib ...
Types of Chemical Reactions
... Combustion Reactions occur when a substance combines with oxygen releasing a large amount of energy in the form of light and heat, heat, it is a combustion reaction. ...
... Combustion Reactions occur when a substance combines with oxygen releasing a large amount of energy in the form of light and heat, heat, it is a combustion reaction. ...
Diazotization-Coupling Reaction--
... A Diazotization—Coupling Reaction: The Preparation of Methyl Orange Formation of a diazonium ion Azote is an old word for nitrogen. Hence, the presence of azo in the name of a chemical implies that nitrogen is present in the structure. Therefore, diazo means two nitrogen atoms. When combined with on ...
... A Diazotization—Coupling Reaction: The Preparation of Methyl Orange Formation of a diazonium ion Azote is an old word for nitrogen. Hence, the presence of azo in the name of a chemical implies that nitrogen is present in the structure. Therefore, diazo means two nitrogen atoms. When combined with on ...
Michael Faraday by Cristian Hunter
... Davy, who had the greatest influence on Faraday's thinking, had shown in 1807 that the metals sodium and potassium can be precipitated from their compounds by an electric current, a process known as electrolysis. Faraday's vigorous pursuit of these experiments led in 1834 to what became known as Far ...
... Davy, who had the greatest influence on Faraday's thinking, had shown in 1807 that the metals sodium and potassium can be precipitated from their compounds by an electric current, a process known as electrolysis. Faraday's vigorous pursuit of these experiments led in 1834 to what became known as Far ...
Spring 2014
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
... (8 pts) If it takes 4.184 J of energy to raise the temperature of exactly one gram of water one degree Celcius, how many photons from this LED are needed to raise the temperature of 250 g of water (about one cup) one degree Celcius? ...
Exam Review Chapter 18-Equilibrium
... 6. What is the effect of adding more CO2 to the following equilibrium reaction? CO2 + H2O↔ H2CO3 a. More H2CO3 is produced. b. More H2O is produced. c. The equilibrium d. No Change 7. Two opposing reactions (A + B ↔C + D) occurring simultaneously at the same rate is an example of: a. reversibility. ...
... 6. What is the effect of adding more CO2 to the following equilibrium reaction? CO2 + H2O↔ H2CO3 a. More H2CO3 is produced. b. More H2O is produced. c. The equilibrium d. No Change 7. Two opposing reactions (A + B ↔C + D) occurring simultaneously at the same rate is an example of: a. reversibility. ...
Acids and bases
... Hydrogen bromide and iodide behave similarly but the extent of ionization of the three hydrogen halides varies along the series: HI > HBr > HCl. This contrasts with the fact that all three compounds are classed as strong acids (i.e. fully ionized) in aqueous solution. Thus, acetic acid exerts a diff ...
... Hydrogen bromide and iodide behave similarly but the extent of ionization of the three hydrogen halides varies along the series: HI > HBr > HCl. This contrasts with the fact that all three compounds are classed as strong acids (i.e. fully ionized) in aqueous solution. Thus, acetic acid exerts a diff ...
Ionic and Covalent Bonding - Fall River Public Schools
... Polar covalent bonds have stronger bonds than non-polar bonds Stronger bonds give higher boiling points (just like ionic compounds) ...
... Polar covalent bonds have stronger bonds than non-polar bonds Stronger bonds give higher boiling points (just like ionic compounds) ...
North Carolina Test of Chemistry RELEASED
... A chemistry student is given 5 samples of a metal. The student measures and records the mass and the volume of each sample and then graphs the data, as shown below. ...
... A chemistry student is given 5 samples of a metal. The student measures and records the mass and the volume of each sample and then graphs the data, as shown below. ...
Shifting Equilibrium
... because changing the temperature changes the relative amounts of reactants and products. Increasing the temperature is, in effect, the addition of energy in the form of heat. According to Le Châtelier’s principle, the stress of the added heat will be lessened by shifting the equilibrium in the direc ...
... because changing the temperature changes the relative amounts of reactants and products. Increasing the temperature is, in effect, the addition of energy in the form of heat. According to Le Châtelier’s principle, the stress of the added heat will be lessened by shifting the equilibrium in the direc ...
IONIZATION METHODS IN MASS SPECTROMETRY
... onding. Smooth channels were obtained by isotropic etching with a solution of H F/HNO3/H2O (20:14:66 v/v). For a 6 min etching time, the channel width was 60 µ m and the depth 25 µm. The channel lengths varied from 3.5 to 5 cm depending on their location on the glass chip. The microchip device was e ...
... onding. Smooth channels were obtained by isotropic etching with a solution of H F/HNO3/H2O (20:14:66 v/v). For a 6 min etching time, the channel width was 60 µ m and the depth 25 µm. The channel lengths varied from 3.5 to 5 cm depending on their location on the glass chip. The microchip device was e ...
CFE Higher Chemistry in Society Homework EB
... starch and potassium iodide solution. The paper changes colour when ozone is present. Ozone reacts with potassium iodide and water to form iodine, oxygen and potassium hydroxide. Write the balanced chemical equation for this reaction. ...
... starch and potassium iodide solution. The paper changes colour when ozone is present. Ozone reacts with potassium iodide and water to form iodine, oxygen and potassium hydroxide. Write the balanced chemical equation for this reaction. ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.