Get Solutions - Iqraa group of institutes
... disease. Such as methemoglobinemia. SO42- : above 500 ppm of SO42- ion in drinking water causes laxative effect otherwise at moderate levels it is harmless F– : Above 2ppm concentration of F– in drinking water cause brown mottling of teeth. ∴ The concentration given in question of SO42- & NO3- in wa ...
... disease. Such as methemoglobinemia. SO42- : above 500 ppm of SO42- ion in drinking water causes laxative effect otherwise at moderate levels it is harmless F– : Above 2ppm concentration of F– in drinking water cause brown mottling of teeth. ∴ The concentration given in question of SO42- & NO3- in wa ...
Chapter 20 Electric Potential and Electric Potential Energy
... Energy conservation: Very important in mechanics. Also very useful in electrostatics Objects in gravitational fields have potential energy (PE). Similarly, Charges in electric fields have ELECTRIC POTENTIAL ENERGY (U) To move an object in a gravitational field requires work. There is a change in the ...
... Energy conservation: Very important in mechanics. Also very useful in electrostatics Objects in gravitational fields have potential energy (PE). Similarly, Charges in electric fields have ELECTRIC POTENTIAL ENERGY (U) To move an object in a gravitational field requires work. There is a change in the ...
Equilibrium Constant
... N2 (g) + 3H2 (g) 2NH3 (g) + 92.22kJ Is the enthalpy (H) Favorable? Is the entropy (S) Favorable? Will this reaction occur spontaneously? Example Problem 17-8 ...
... N2 (g) + 3H2 (g) 2NH3 (g) + 92.22kJ Is the enthalpy (H) Favorable? Is the entropy (S) Favorable? Will this reaction occur spontaneously? Example Problem 17-8 ...
AP Chemistry
... solute and solvent increases disorder, but formation of hydration bonds between solute and solvent decreases disorder, therefore it is impossible to predict the sign for S (although most dissolving is +S. All chemical reactions that result in more moles of gas products compared to reactants have a ...
... solute and solvent increases disorder, but formation of hydration bonds between solute and solvent decreases disorder, therefore it is impossible to predict the sign for S (although most dissolving is +S. All chemical reactions that result in more moles of gas products compared to reactants have a ...
Subject Materials for Chemistry
... 7. A baker found that the cake prepared by him is hard and small in size. Which ingredient has he forgotten to add that would have made the cake Fluffy? Give reason. Ans: Baker forgotten to add NaHCO3, Baking soda. NaHCO3 in the form of baking powder is added to dough for preparing cake or bread. Na ...
... 7. A baker found that the cake prepared by him is hard and small in size. Which ingredient has he forgotten to add that would have made the cake Fluffy? Give reason. Ans: Baker forgotten to add NaHCO3, Baking soda. NaHCO3 in the form of baking powder is added to dough for preparing cake or bread. Na ...
Gibbs Free Energy - nchsdduncanchem2
... respectively. This shouldn't be a surprise, because that's what they were originally named in the first place! Just keep in mind that some outdated or unsophisticated texts might still use the pseudonyms mentioned above (guised as, say, the title of a module). Gibbs Energy The Gibbs Energy is named ...
... respectively. This shouldn't be a surprise, because that's what they were originally named in the first place! Just keep in mind that some outdated or unsophisticated texts might still use the pseudonyms mentioned above (guised as, say, the title of a module). Gibbs Energy The Gibbs Energy is named ...
Chemistry 20 Lesson 36 – The Whole Enchilada
... The boiling point of fluorine is significantly less than that of chlorine. ...
... The boiling point of fluorine is significantly less than that of chlorine. ...
Group 1: The Alkali Metals
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
`A` LEVEL H2 CHEMISTRY ORGANIC REACTIONS SUMMARY By
... (a) explain that some chemical reactions are accompanied by energy changes, principally in the form of heat energy; the energy changes can be exothermic (∆H negative) or endothermic (∆H positive) (b) explain and use the terms: (i) enthalpy change of reaction and standard conditions, with particular ...
... (a) explain that some chemical reactions are accompanied by energy changes, principally in the form of heat energy; the energy changes can be exothermic (∆H negative) or endothermic (∆H positive) (b) explain and use the terms: (i) enthalpy change of reaction and standard conditions, with particular ...
The absorption spectra of very small CdS or ZnS particles (1
... made with zinc sulfide some fifty years ago (Ref. 1,2). Zinc sulfide, a component of the white pigment lithophone, was shown not only to photolyse but also to catalyse redox processes including the photo-decomposition of water. The catalysis of redox reactions has also been observed in colloid chemi ...
... made with zinc sulfide some fifty years ago (Ref. 1,2). Zinc sulfide, a component of the white pigment lithophone, was shown not only to photolyse but also to catalyse redox processes including the photo-decomposition of water. The catalysis of redox reactions has also been observed in colloid chemi ...
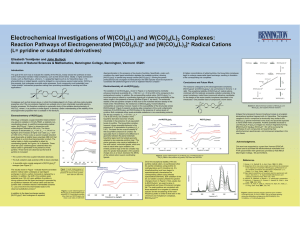
Electrochemical Investigations of W(CO) (L) and W(CO) (L) Complexes:
... Complexes such as that shown above, in which the bridging ligand is 4,4'-bpy, will show redox-tunable properties only if the non-emissive fragment can undergo one or more chemically reversible electrontransfer processes. We therefore undertook a systematic study of the oxidative electrochemistry of ...
... Complexes such as that shown above, in which the bridging ligand is 4,4'-bpy, will show redox-tunable properties only if the non-emissive fragment can undergo one or more chemically reversible electrontransfer processes. We therefore undertook a systematic study of the oxidative electrochemistry of ...
Chem101 - Lecture 5 Introduction Introduction
... reactions that require the electrons flowing from the oxidation reaction to the reduction reaction to flow through a wire. ...
... reactions that require the electrons flowing from the oxidation reaction to the reduction reaction to flow through a wire. ...
The Bio-Organometallic Chemistry of Technetium and Rhenium
... metal can readily change its oxidation and coordination numbers makes it difficult to develop high yielding reactions that result in the formation of only a single compound. This is made even more difficult in radiopharmaceutical chemistry where reactions are performed in water under highly dilute r ...
... metal can readily change its oxidation and coordination numbers makes it difficult to develop high yielding reactions that result in the formation of only a single compound. This is made even more difficult in radiopharmaceutical chemistry where reactions are performed in water under highly dilute r ...
Atomic Structure
... Atoms of magnesium are neutral because they contain the same number of electrons and .................. ...
... Atoms of magnesium are neutral because they contain the same number of electrons and .................. ...
Chemistry
... To know: What a chemical reaction is, the scheme of reaction, the chemical equation. The laws of conservation of mass of substances in a course of chemical reactions, the volume ratios of gases in chemical reactions. External effects that accompany chemical reactions. The concept of oxidizing agent, ...
... To know: What a chemical reaction is, the scheme of reaction, the chemical equation. The laws of conservation of mass of substances in a course of chemical reactions, the volume ratios of gases in chemical reactions. External effects that accompany chemical reactions. The concept of oxidizing agent, ...
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Press here to hemy 102 lab manual
... chemical bond between them. There are three types of chemical bonds: ionic, covalent, and metallic. The term ionic bond refers to electrostatic forces that exist between ions of opposite charge. A covalent bond results from the sharing of electrons between two atoms. The more familiar examples of co ...
... chemical bond between them. There are three types of chemical bonds: ionic, covalent, and metallic. The term ionic bond refers to electrostatic forces that exist between ions of opposite charge. A covalent bond results from the sharing of electrons between two atoms. The more familiar examples of co ...
Chapter 2 - Phillips Scientific Methods
... • An anion is a negatively - charged ion. • An ionic bond is an attraction between an anion and a cation. • Compounds formed by ionic bonds are called ionic compounds, or salts. • Salts, such as sodium chloride (table salt), are often found in nature as crystals. Copyright © 2008 Pearson Education, ...
... • An anion is a negatively - charged ion. • An ionic bond is an attraction between an anion and a cation. • Compounds formed by ionic bonds are called ionic compounds, or salts. • Salts, such as sodium chloride (table salt), are often found in nature as crystals. Copyright © 2008 Pearson Education, ...
PHYSICAL SETTING CHEMISTRY
... (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
... (3) gain electrons and have a decrease in oxidation number (4) gain electrons and have an increase in oxidation number ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.