JF Physical Chemistry 2010-2011. JF CH 1101: Introduction to
... Answer either : part (a) and part (b) or part (c) and part (d). a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed b ...
... Answer either : part (a) and part (b) or part (c) and part (d). a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed b ...
TDR XFEL workshop series Atomic, molecular and cluster physics
... Recently, the interest for experimental and theoretical investigations of interaction processes of x-ray radiation with atom has increased [1,2]. Similar investigations are very important for modern fundamental and applied physics. So, the explorations of non-linear processes of the x-ray photons in ...
... Recently, the interest for experimental and theoretical investigations of interaction processes of x-ray radiation with atom has increased [1,2]. Similar investigations are very important for modern fundamental and applied physics. So, the explorations of non-linear processes of the x-ray photons in ...
Grade 8 th Science Curriculum Scope and Sequence
... Recognize that compounds can be represented by symbols. Provide data from investigations to support the fact that energy is transformed during chemical reactions. Provide examples to explain the difference between a physical change and a chemical change. ...
... Recognize that compounds can be represented by symbols. Provide data from investigations to support the fact that energy is transformed during chemical reactions. Provide examples to explain the difference between a physical change and a chemical change. ...
MS PowerPoint - Catalysis Eprints database
... In homogeneous as well as heterogeneous catalysts such active sites are normally referred to as vacant site/ co-ordinatively unsaturated site (cus). Substrates on adsorption at cus get activated In a typical homogeneous catalyst the active site is a cus in a metal complex In heterogeneous catalysis, ...
... In homogeneous as well as heterogeneous catalysts such active sites are normally referred to as vacant site/ co-ordinatively unsaturated site (cus). Substrates on adsorption at cus get activated In a typical homogeneous catalyst the active site is a cus in a metal complex In heterogeneous catalysis, ...
Review Questions for 1st year chemistry
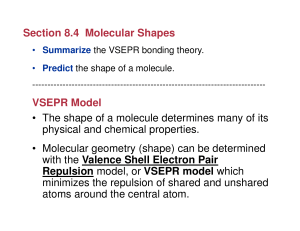
... Answer: C Electronegativity decreases as you move down the periodic table because as you move down, there are more valence shells which shield the pull of the protons in the nucleus. Fluorine is the first element in its group and therefore has the highest electronegativity. ...
... Answer: C Electronegativity decreases as you move down the periodic table because as you move down, there are more valence shells which shield the pull of the protons in the nucleus. Fluorine is the first element in its group and therefore has the highest electronegativity. ...
chemistry I review pwrpt.
... •Each path has a specific energy requirement. •These circular paths are called energy levels. •Limited number of electrons on each http://micro.magnet.fsu.edu/ energy level. ...
... •Each path has a specific energy requirement. •These circular paths are called energy levels. •Limited number of electrons on each http://micro.magnet.fsu.edu/ energy level. ...
Document
... • Add together the reduction halfreaction with the oxidation halfreaction to get the complete redox reaction. ...
... • Add together the reduction halfreaction with the oxidation halfreaction to get the complete redox reaction. ...
Electronic Structure and the Periodic Table
... When putting electrons into orbitals with the same energy, place one electron in each orbital before pairing them up. The lone electrons will have the same direction of spin. The existence of unpaired electrons can be tested for ...
... When putting electrons into orbitals with the same energy, place one electron in each orbital before pairing them up. The lone electrons will have the same direction of spin. The existence of unpaired electrons can be tested for ...