communication - Kyushu University Library
... because the other alcohols which does not act as alkylating agents and the surfactant brought a similar effect to 2-choroethanol although the alkylation cannot be excluded completely. Therefore, it is reasonable that the structure of the cation part of the catalyst remains intact. A stoichiometric r ...
... because the other alcohols which does not act as alkylating agents and the surfactant brought a similar effect to 2-choroethanol although the alkylation cannot be excluded completely. Therefore, it is reasonable that the structure of the cation part of the catalyst remains intact. A stoichiometric r ...
18.3 Standard Entropies and the Third Law of
... Numbers in parentheses after definitions give the text section in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. thermodynamics* study of the relationship ...
... Numbers in parentheses after definitions give the text section in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. thermodynamics* study of the relationship ...
Synthesis and Characterization of Tetradentate Complexes Type
... coordination modes of oxime and oximato sp ecies indicated a versatile electronic distribution within the ligand. This, in turn suggested that the chemistry of metal-bonded oximes should be rich. The inspection of data accumulated in the literature confirmed these assumptions (2).Transition metal co ...
... coordination modes of oxime and oximato sp ecies indicated a versatile electronic distribution within the ligand. This, in turn suggested that the chemistry of metal-bonded oximes should be rich. The inspection of data accumulated in the literature confirmed these assumptions (2).Transition metal co ...
CB document - mvhs
... involve an energy change in the form of heat—heat released as the result of a reaction, or heat absorbed as a reaction proceeds. Energy changes accompany all chemical reactions and are due to rearranging of chemical bonding. The energy of chemical bonding, a form of chemical potential energy, should ...
... involve an energy change in the form of heat—heat released as the result of a reaction, or heat absorbed as a reaction proceeds. Energy changes accompany all chemical reactions and are due to rearranging of chemical bonding. The energy of chemical bonding, a form of chemical potential energy, should ...
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
Alkyl Halides SN and E reactions
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
... Substitution and elimination compete. In 3° and 2° alkyl halides, E2 is faster. In 1° and Me° alkyl halides, SN2 occurs. 3. Weakly basic, weak nucleophiles, like H2O, EtOH, CH3COOH, etc., cannot react unless a C+ forms. This only occurs with 2° or 3° substrates. Once the C+ forms, both SN1 and E1 oc ...
base hydrolysis of cobalt(iii)
... I was pleased to be invited by Professor D. A. Davenport to present a paper at the symposium C. K. Ingold: Master and Mandarin of Physical Organic Chemistry to honor Professor Sir Christopher Ingold on the centennial year of his birth. The chemistry community recalls that he was one of the giants of ...
... I was pleased to be invited by Professor D. A. Davenport to present a paper at the symposium C. K. Ingold: Master and Mandarin of Physical Organic Chemistry to honor Professor Sir Christopher Ingold on the centennial year of his birth. The chemistry community recalls that he was one of the giants of ...
Notes for Quarter I
... refraction in a number of ways; for example, a straw in a glass of water appears bent because light is moving from the medium of air to water (see also Fig. 6, p. 648). White light passing through a prism can be separated into its component colors because of refraction also (Fig. 7, p. 648). Diffra ...
... refraction in a number of ways; for example, a straw in a glass of water appears bent because light is moving from the medium of air to water (see also Fig. 6, p. 648). White light passing through a prism can be separated into its component colors because of refraction also (Fig. 7, p. 648). Diffra ...
Group 1: The Alkali Metals
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
... their electrons in reactions and often have an oxidation state of +1. These metals are characterized as being extremely soft and silvery in color. They also have low boiling and melting points and are less dense than most elements. Li, Na, and K float on water because of their low densities. All of ...
Ghw#8-chapter-17-Tro-F-16
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
DCY1B - Manonmaniam Sundaranar University
... the decrease in atomic radii is much less compared to P-block elements. This is because, addition of electrons to the inner (n-1) d orbital effectively screen the outer ns electron from the added nuclear charge (primary screening effect). Further, the decrease in the atomic radii is minimum for grou ...
... the decrease in atomic radii is much less compared to P-block elements. This is because, addition of electrons to the inner (n-1) d orbital effectively screen the outer ns electron from the added nuclear charge (primary screening effect). Further, the decrease in the atomic radii is minimum for grou ...
chemical equilibrium
... As the rate of reaction is dependant on the concentration of reactants... • the forward reaction starts off fast but slows as the reactants get less concentrated • initially, there is no backward reaction but, as products form, it will get faster • provided the temperature remains constant there wil ...
... As the rate of reaction is dependant on the concentration of reactants... • the forward reaction starts off fast but slows as the reactants get less concentrated • initially, there is no backward reaction but, as products form, it will get faster • provided the temperature remains constant there wil ...
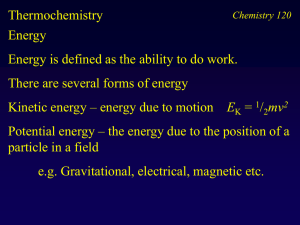
Chemistry 120
... U is a function of the state of the material only, not of the history of the sample or the path taken to prepare the state of the sample. Heat is the transfer of energy between the surroundings and the sample - the symbol for heat is q Work is the result of a force acting over a distance - the symbo ...
... U is a function of the state of the material only, not of the history of the sample or the path taken to prepare the state of the sample. Heat is the transfer of energy between the surroundings and the sample - the symbol for heat is q Work is the result of a force acting over a distance - the symbo ...
Electrophilic Aromatic Substitution and Substituted Benzenes
... • To understand how substituents activate or deactivate the ring, we must consider the first step in electrophilic aromatic substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the ...
... • To understand how substituents activate or deactivate the ring, we must consider the first step in electrophilic aromatic substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the ...
Document
... • To understand how substituents activate or deactivate the ring, we must consider the first step in electrophilic aromatic substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the ...
... • To understand how substituents activate or deactivate the ring, we must consider the first step in electrophilic aromatic substitution. • The first step involves addition of the electrophile (E+) to form a resonance stabilized carbocation. • The Hammond postulate makes it possible to predict the ...
fference: mechanistic How phenyl makes a di insights into the ruthenium( )-catalysed
... insights from DFT and experiments also allowed for the design of a protocol that expands the scope of ...
... insights from DFT and experiments also allowed for the design of a protocol that expands the scope of ...