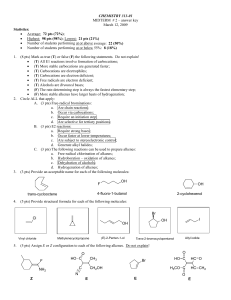
Chapter 4 Lecture Notes in PowerPoint
... drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum number of pizzas we actuall ...
... drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum number of pizzas we actuall ...
Photo-induced metal–ligand bond weakening, potential
... significantly with the metal are empty) then the three metal orbitals are part of p bonding molecular orbitals. If no ligand p orbitals are available (amine ligands, for example) then these d orbitals are nonbonding. In the examples discussed here, the only p interacting ligands are those with fille ...
... significantly with the metal are empty) then the three metal orbitals are part of p bonding molecular orbitals. If no ligand p orbitals are available (amine ligands, for example) then these d orbitals are nonbonding. In the examples discussed here, the only p interacting ligands are those with fille ...
Appendix N CONCENTRATION UNITS
... knew that if carbon (charcoal) was mixed with iron oxides and heated, elemental iron and carbon dioxide would be formed. This practical knowledge was attained without any concept of atoms, molecules and reactions. By the nineteenth century, the study of stoichiometry allowed chemists to determine ma ...
... knew that if carbon (charcoal) was mixed with iron oxides and heated, elemental iron and carbon dioxide would be formed. This practical knowledge was attained without any concept of atoms, molecules and reactions. By the nineteenth century, the study of stoichiometry allowed chemists to determine ma ...
First Law of Thermodynamics
... the reactants and products, not on the manner in which they react! This is an important property because it allows us to determine ΔE for a reaction using any path - even one that is unreasonable - as long as the reactants and products remain the same. Both q and w are path dependent, so they are no ...
... the reactants and products, not on the manner in which they react! This is an important property because it allows us to determine ΔE for a reaction using any path - even one that is unreasonable - as long as the reactants and products remain the same. Both q and w are path dependent, so they are no ...
On the Convergence of Atomic Charges with the Size of the
... for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid dissociation (pKa) can be related to the charge of the conjugate base2-6. Further, atomic charges ca ...
... for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid dissociation (pKa) can be related to the charge of the conjugate base2-6. Further, atomic charges ca ...
ACTIVIDAD CATALÍTICA DE COMPUESTOS COMPLEJOS DE Pd (II)
... square planar geometry is specially favoured. These square planar complexes, particularly those from the second row, have proved to be active species for the catalytic hydrogenation of multiple bonds and other reactions [12]. They are important in catalysis since the metal atom can increase its coor ...
... square planar geometry is specially favoured. These square planar complexes, particularly those from the second row, have proved to be active species for the catalytic hydrogenation of multiple bonds and other reactions [12]. They are important in catalysis since the metal atom can increase its coor ...
16 Chemical Equilibrium Chapter Outline Rates of Reaction
... Copyright © 2014 John Wiley & Sons, Inc. All rights reserved. ...
... Copyright © 2014 John Wiley & Sons, Inc. All rights reserved. ...
Ch 17 Equilibrium
... • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to the rate of the reverse reac ...
... • Consider colorless frozen N2O4. At room temperature, it decomposes to brown NO2: N2O4(g) 2NO2(g). • At some time, the color stops changing and we have a mixture of N2O4 and NO2. • Chemical equilibrium is the point at which the rate of the forward reaction is equal to the rate of the reverse reac ...
communication - Kyushu University Library
... because the other alcohols which does not act as alkylating agents and the surfactant brought a similar effect to 2-choroethanol although the alkylation cannot be excluded completely. Therefore, it is reasonable that the structure of the cation part of the catalyst remains intact. A stoichiometric r ...
... because the other alcohols which does not act as alkylating agents and the surfactant brought a similar effect to 2-choroethanol although the alkylation cannot be excluded completely. Therefore, it is reasonable that the structure of the cation part of the catalyst remains intact. A stoichiometric r ...