chapter 8 - Denton ISD
... equations. As you can see, some things can be shown in different ways. For example, sometimes a gaseous product is indicated by an arrow pointing upward,↑, instead of (g). A downward arrow, ↓, is often used to show the formation of a precipitate during a reaction in solution. The conditions under wh ...
... equations. As you can see, some things can be shown in different ways. For example, sometimes a gaseous product is indicated by an arrow pointing upward,↑, instead of (g). A downward arrow, ↓, is often used to show the formation of a precipitate during a reaction in solution. The conditions under wh ...
5 Energetics - Pearson Schools and FE Colleges
... during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electr ...
... during respiration, when glucose reacts with oxygen. Modern lifestyles are dependent on the transfer of energy that occurs when fuels burn. As we explore the source of these energy changes, we will deepen our understanding of why bonds are broken and formed during a chemical reaction, and why electr ...
Efficient Homogeneous Catalysis in the Reduction of CO to CO
... Considerably higher turnover numbers are achieved at higher reaction temperatures. Turnover of pinacolborate 2 presumably occurs much more rapidly; the boryl complex 1, generated in situ, is sufficiently stable toward decomposition to react productively with CO2. The reduction of CO2 at 100 °C, usin ...
... Considerably higher turnover numbers are achieved at higher reaction temperatures. Turnover of pinacolborate 2 presumably occurs much more rapidly; the boryl complex 1, generated in situ, is sufficiently stable toward decomposition to react productively with CO2. The reduction of CO2 at 100 °C, usin ...
Beginning Chemistry
... and heterogeneous mixtures are sometimes called simply mixtures. In heterogeneous mixtures, it is possible to see differences in the sample, merely by looking, although a microscope may be required. In contrast, homogeneous mixtures look the same throughout the sample, even under the best optical mi ...
... and heterogeneous mixtures are sometimes called simply mixtures. In heterogeneous mixtures, it is possible to see differences in the sample, merely by looking, although a microscope may be required. In contrast, homogeneous mixtures look the same throughout the sample, even under the best optical mi ...
Study guide for final
... 42) A saturated solution holds the maximum amount of solute under the solution conditions. 43) One liter of 6.0 M HNO3 contains the same number of H+ ions as does one liter of 6.0 M H2SO4. 44) A 1.0 M [H3O+] solution of a strong acid would have a pH equal to zero. 45) A solution that has a pH of 8.5 ...
... 42) A saturated solution holds the maximum amount of solute under the solution conditions. 43) One liter of 6.0 M HNO3 contains the same number of H+ ions as does one liter of 6.0 M H2SO4. 44) A 1.0 M [H3O+] solution of a strong acid would have a pH equal to zero. 45) A solution that has a pH of 8.5 ...
File
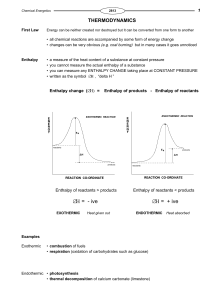
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
Chapter 5 powerpoint
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
Thermochemistry
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
... Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry ...
The Physical Chemistry, Theory and Technique of
... Any of the colligative properties may be used to indicate osmolality. There are advantages and disadvantages to each one. 1. Vapor pressure. This technique allows the measurement to be made at the temperature desired. It is more complicated, however, not as reproducible in the biological range, and ...
... Any of the colligative properties may be used to indicate osmolality. There are advantages and disadvantages to each one. 1. Vapor pressure. This technique allows the measurement to be made at the temperature desired. It is more complicated, however, not as reproducible in the biological range, and ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Multiple-choice questions : 1. The following graph shows the volume
... 10. Hydrocarbons undergo complete combustion to give carbon dioxide under sufficient oxygen supply. Toxic carbon monoxide is produced if the oxygen supply is insufficient. Catalytic converters are usually installed in vehicles to minimize the emission of carbon monoxide in exhaust gas. (a) (i) Bala ...
... 10. Hydrocarbons undergo complete combustion to give carbon dioxide under sufficient oxygen supply. Toxic carbon monoxide is produced if the oxygen supply is insufficient. Catalytic converters are usually installed in vehicles to minimize the emission of carbon monoxide in exhaust gas. (a) (i) Bala ...