![C2_revision_slides_V3_+_questions_+_MS_-_H[1]](http://s1.studyres.com/store/data/000092833_1-97fb33725e7f1ef12029ed42751d3dca-300x300.png)
Ionic bonding
... 2. What in the name and formula of the acid that can be used to make magnesium chloride from magnesium ribbon? 3. What is the definition of an acid? 4. What is the difference between an alkali and a base? 5. What gas is formed when an acid reacts with a metal? 6. How can we test for this gas? 7. Wha ...
... 2. What in the name and formula of the acid that can be used to make magnesium chloride from magnesium ribbon? 3. What is the definition of an acid? 4. What is the difference between an alkali and a base? 5. What gas is formed when an acid reacts with a metal? 6. How can we test for this gas? 7. Wha ...
Document
... The standard entropy of a substance—its absolute entropy, S°—is the entropy value for the standard state of the species. The standard state is indicated with the superscript degree sign. For a pure substance, its standard state is 1 atm pressure. For a substance in solution, its standard state is a ...
... The standard entropy of a substance—its absolute entropy, S°—is the entropy value for the standard state of the species. The standard state is indicated with the superscript degree sign. For a pure substance, its standard state is 1 atm pressure. For a substance in solution, its standard state is a ...
Introduction to Nanoscience
... A nanodevice that often appears in science fiction is a nanocamera. This is used to view the inside of the body or in other confined spaces where an ordinary camera would not fit. Unfortunately, it is not possible to make such a camera using conventional far field optics. Light sources and light det ...
... A nanodevice that often appears in science fiction is a nanocamera. This is used to view the inside of the body or in other confined spaces where an ordinary camera would not fit. Unfortunately, it is not possible to make such a camera using conventional far field optics. Light sources and light det ...
Lab 2 - Academic Computer Center
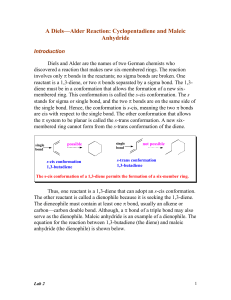
... We saw in previous lessons that some groups are electron donating (EDG) and some are electron withdrawing (EWG). The manner of substitution of electron-donating and electron-withdrawing groups in the reactants governs how easily the reactants produce a Diels-Alder product. When you give someone dir ...
... We saw in previous lessons that some groups are electron donating (EDG) and some are electron withdrawing (EWG). The manner of substitution of electron-donating and electron-withdrawing groups in the reactants governs how easily the reactants produce a Diels-Alder product. When you give someone dir ...
homework-11th-chem
... 92 Express the change in internal energy of a system when(i) No heat is absorbed by the system from the surroundings, but work (w) is done on the system. What type of wall does the system have?(ii) No work is done on the system, but q amount of heat is taken out from the system and given to the sur ...
... 92 Express the change in internal energy of a system when(i) No heat is absorbed by the system from the surroundings, but work (w) is done on the system. What type of wall does the system have?(ii) No work is done on the system, but q amount of heat is taken out from the system and given to the sur ...
Thermodynamics
... -If G<0, the reaction is spontaneous (forward dir.) -If G>0, the reaction is not spontaneous (forward dir.) -If G=0,the reaction is at equilibrium -A reaction is spontaneous in the forward direction only if ΔG is negative. -Spontaneity is controlled by enthalpy and entropy. If ΔH (-) & ΔS (+),the ...
... -If G<0, the reaction is spontaneous (forward dir.) -If G>0, the reaction is not spontaneous (forward dir.) -If G=0,the reaction is at equilibrium -A reaction is spontaneous in the forward direction only if ΔG is negative. -Spontaneity is controlled by enthalpy and entropy. If ΔH (-) & ΔS (+),the ...
Methane - ARZELORIVAS IS
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
... This reaction has the following characteristic properties. It doesn't take place in the dark or at low temperatures. It occurs in the presence of ultraviolet light or at temperatures above 250oC. Once the reaction gets started, it continues after the light is turned off. The products of the ...
2007 local exam - American Chemical Society
... This test is designed to be taken with an answer sheet on which the student records his or her responses. All answers are to be marked on that sheet, not written in the booklet. Each student should be provided with an answer sheet and scratch paper, both of which must be turned in with the test book ...
... This test is designed to be taken with an answer sheet on which the student records his or her responses. All answers are to be marked on that sheet, not written in the booklet. Each student should be provided with an answer sheet and scratch paper, both of which must be turned in with the test book ...
Chapter 7 Chemical Reactions
... There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to classify chemical reactions, which allows chemist to recognize patterns and predict the products of reactions One of these ...
... There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to classify chemical reactions, which allows chemist to recognize patterns and predict the products of reactions One of these ...
Unit Two Objectives
... GHS Chemistry Final exam Review Sheet 2014 These physical properties are INTENSIVE, which means that the property does not depend on the amount of material present. Physical/Intensive Properties include Boiling Point, Melting Point, Freezing Point, Color, Odor, State, malleability, Ductility (metal ...
... GHS Chemistry Final exam Review Sheet 2014 These physical properties are INTENSIVE, which means that the property does not depend on the amount of material present. Physical/Intensive Properties include Boiling Point, Melting Point, Freezing Point, Color, Odor, State, malleability, Ductility (metal ...
Chapter 9
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
... Three (or more) atom molecules cannot be explained by simple overlap of orbitals. Fact: a bond generally forms between two half-filled orbitals. Fact: an s-type orbital is spherical, so it could form a bond in any direction. Fact: the three p-type orbitals are at 90 degree angles to each other. ...
H3AsO4 + 3 I- + 2 H3O+ H3AsO3 + I3- + H2O
... Bond strength and length is also affected by the number of shared electrons. Sharing of one pair of electrons produces a single bond; whereas the sharing of two or three pairs of electrons produces double or triple bonds, respectively. Multiple bonds are stronger and shorter than single bonds. The p ...
... Bond strength and length is also affected by the number of shared electrons. Sharing of one pair of electrons produces a single bond; whereas the sharing of two or three pairs of electrons produces double or triple bonds, respectively. Multiple bonds are stronger and shorter than single bonds. The p ...