Types of reactions you know:
... It’s sometimes helpful to group reactions according to what they are useful for in synthesis: 1) Adding alkyl groups – if the product has more carbons than the starting material. a. Add a primary alkyl halide to a terminal alkyne (an SN2 rxn) ...
... It’s sometimes helpful to group reactions according to what they are useful for in synthesis: 1) Adding alkyl groups – if the product has more carbons than the starting material. a. Add a primary alkyl halide to a terminal alkyne (an SN2 rxn) ...
Chemistry: The Study of Change
... • I believe Entropy supports creationism. Saying that as time goes on things get more chaotic. This implies that at one time everything was in perfect order. • All science has shown the universe is constantly expanding, spreading out from a common origin (increasing entropy). • The Big bang theory d ...
... • I believe Entropy supports creationism. Saying that as time goes on things get more chaotic. This implies that at one time everything was in perfect order. • All science has shown the universe is constantly expanding, spreading out from a common origin (increasing entropy). • The Big bang theory d ...
chemical equation - Central Lyon CSD
... numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it. ...
... numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it. ...
Charge transport in a polypeptide chain
... molecule and chemical reaction far away at the other end of a molecule is not treated in the conventional theory of reaction kinetics [6]. Traditional reaction rate theory is local, where chemical reaction proceeds on the site of excitation – we might refer to this as proximal kinetics. In contrast ...
... molecule and chemical reaction far away at the other end of a molecule is not treated in the conventional theory of reaction kinetics [6]. Traditional reaction rate theory is local, where chemical reaction proceeds on the site of excitation – we might refer to this as proximal kinetics. In contrast ...
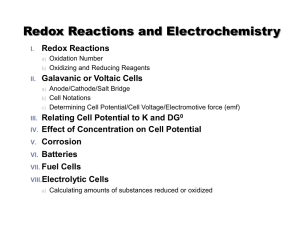
Redox Reactions
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...
PowerPoint **
... Draw mechanisms for the following reaction and explain why carbonyl acid can’t undergo similar reaction ...
... Draw mechanisms for the following reaction and explain why carbonyl acid can’t undergo similar reaction ...
February 13, 2008
... A. At equilibrium, the total concentration of products equals the total concentration of reactants B. Equilibrium is the result of the cessation of all chemical change. C. There is only one set of equilibrium concentrations that equals the Kc value. D. The rate constant of the forward reaction is eq ...
... A. At equilibrium, the total concentration of products equals the total concentration of reactants B. Equilibrium is the result of the cessation of all chemical change. C. There is only one set of equilibrium concentrations that equals the Kc value. D. The rate constant of the forward reaction is eq ...
- Deans Community High School
... B. lowers the energy which molecules need for successful collisions C. provides energy so that more molecules have successful collisions D. form bonds with reacting molecules 3. Which of the following graphs of rate of reaction against temperature would apply to the neutralisation of dilute hydrochl ...
... B. lowers the energy which molecules need for successful collisions C. provides energy so that more molecules have successful collisions D. form bonds with reacting molecules 3. Which of the following graphs of rate of reaction against temperature would apply to the neutralisation of dilute hydrochl ...
Document
... • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. • Energy can be converted from one type into another. ...
... • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. • Energy can be converted from one type into another. ...
Isolating High Value Aromatics from Lignin Stockpiles
... (transportable) crude yields. Lignin is a polymer found in woody plants bound to cellulose and hemicellulose. It is produced as a biomass waste from the paper milling industry and a majority is inefficiently burned as fuel.2 However, lignin contains many aromatics and has the potential to produce hi ...
... (transportable) crude yields. Lignin is a polymer found in woody plants bound to cellulose and hemicellulose. It is produced as a biomass waste from the paper milling industry and a majority is inefficiently burned as fuel.2 However, lignin contains many aromatics and has the potential to produce hi ...
Slide 1
... *To understand Friedlaender synthesis. *To show the equation & the mechanism of the reaction. *To gain some knowledge about the applications & recent literatures related to the reaction. ...
... *To understand Friedlaender synthesis. *To show the equation & the mechanism of the reaction. *To gain some knowledge about the applications & recent literatures related to the reaction. ...
Paired with Lecture
... If DG is negative then there is a probability that a reaction will occur. The more negative DG becomes, the more driving force there is for the reaction Thermodynamics tells us the probability of a reaction but not the rate – the rate of a reaction is determined by Kinetics ...
... If DG is negative then there is a probability that a reaction will occur. The more negative DG becomes, the more driving force there is for the reaction Thermodynamics tells us the probability of a reaction but not the rate – the rate of a reaction is determined by Kinetics ...
Bulent Terem - CH324 - Syllabus | Chaminade
... submitted before the exam day or within 24 hours of the date and time of the exam accompanied by a written verification. Letter grades are assigned on the basis of the following scale: A > 85 B ...
... submitted before the exam day or within 24 hours of the date and time of the exam accompanied by a written verification. Letter grades are assigned on the basis of the following scale: A > 85 B ...
Physical Organic Chemistry
... Elimination Reactions Elimination reaction: to eliminate two atoms, two groups, or one atom and one group without substituted with another atom or group. The elimination of HX molecule from alkyl derivatives. While X is a halogen or ester… etc. the hydrogen atom on adjacent carbon with X Elim ...
... Elimination Reactions Elimination reaction: to eliminate two atoms, two groups, or one atom and one group without substituted with another atom or group. The elimination of HX molecule from alkyl derivatives. While X is a halogen or ester… etc. the hydrogen atom on adjacent carbon with X Elim ...