1aUnit Two Handouts - Dunmore High School
... If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this out when writing net ionic equations.) ...
... If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this out when writing net ionic equations.) ...
Chapter-1-Intro - Mister Chemistry Welcomes You!
... a substance is form of matter that has a definite or constant composition and distinct properties, for example water ammonia table sugar gold oxygen ...
... a substance is form of matter that has a definite or constant composition and distinct properties, for example water ammonia table sugar gold oxygen ...
Spring 2013 Semester Exam Study Guide (Bonding, Nomenclature
... ____ 96. In the word equation, sodium oxide + water sodium hydroxide, the formula for sodium hydroxide is represented by a. Na2OH. c. NaO2. b. NaOH. d. Na2O. ____ 97. Which word equation represents the reaction that produces water from hydrogen and oxygen? a. Water is produced from hydrogen and ox ...
... ____ 96. In the word equation, sodium oxide + water sodium hydroxide, the formula for sodium hydroxide is represented by a. Na2OH. c. NaO2. b. NaOH. d. Na2O. ____ 97. Which word equation represents the reaction that produces water from hydrogen and oxygen? a. Water is produced from hydrogen and ox ...
Grade 11 Review Package
... always subtract the smaller electronegativity from the larger one. Figure R.8 illustrates the range in bond character for different values of ∆EN. ...
... always subtract the smaller electronegativity from the larger one. Figure R.8 illustrates the range in bond character for different values of ∆EN. ...
Bellin College Homework Supplement
... 61. Sodium chlorite, NaClO2, is a component of mouthwashes, toothpastes, and contact lens cleaning solutions. Draw the Lewis structure for the chlorite ion, ...
... 61. Sodium chlorite, NaClO2, is a component of mouthwashes, toothpastes, and contact lens cleaning solutions. Draw the Lewis structure for the chlorite ion, ...
CHEMISTRY 110 LECTURE
... 6. Iron (III) oxide can react with aluminum metal to produce aluminum oxide and iron metal (hint: this is the chemical rxn!!) This is called the thermit reaction and it produces so much heat that it can be used for incendiary bombs and for welding. How many grams of aluminum oxide will be produced b ...
... 6. Iron (III) oxide can react with aluminum metal to produce aluminum oxide and iron metal (hint: this is the chemical rxn!!) This is called the thermit reaction and it produces so much heat that it can be used for incendiary bombs and for welding. How many grams of aluminum oxide will be produced b ...
syllabus details - hrsbstaff.ednet.ns.ca
... Cross reference with topics 2, 4 and 5. Data for all these properties are listed in the data booklet. Explanations for the first four trends should be given in terms of the balance between the attraction of the nucleus for the electrons and the repulsion between electrons. Explanations based on effe ...
... Cross reference with topics 2, 4 and 5. Data for all these properties are listed in the data booklet. Explanations for the first four trends should be given in terms of the balance between the attraction of the nucleus for the electrons and the repulsion between electrons. Explanations based on effe ...
Section A oxide in molten cryolite?
... Q1 In the extraction of aluminium by electrolysis, why is it necessary to dissolve aluminium oxide in molten cryolite? A to reduce the very high melting point of the electrolyte B cryolite provides the ions needed to carry the current C cryolite reacts with the aluminium oxide to form ions D molten ...
... Q1 In the extraction of aluminium by electrolysis, why is it necessary to dissolve aluminium oxide in molten cryolite? A to reduce the very high melting point of the electrolyte B cryolite provides the ions needed to carry the current C cryolite reacts with the aluminium oxide to form ions D molten ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
3.1.1.2 Mass number and isotopes
... In contrast with kinetics, which is a study of how quickly reactions occur, a study of equilibria indicates how far reactions will go. Le Chatelier’s principle can be used to predict the effects of changes in temperature, pressure and concentration on the yield of a reversible reaction. This has imp ...
... In contrast with kinetics, which is a study of how quickly reactions occur, a study of equilibria indicates how far reactions will go. Le Chatelier’s principle can be used to predict the effects of changes in temperature, pressure and concentration on the yield of a reversible reaction. This has imp ...
H 2
... the Law of Conservation of Matter If an equation isn’t balanced, you may miss a product that is not easily observed. An equation is a chemical recipe. If it isn’t balanced, it is like a recipe that doesn’t include any amounts. It would be useless to try to make something from such a recipe. ...
... the Law of Conservation of Matter If an equation isn’t balanced, you may miss a product that is not easily observed. An equation is a chemical recipe. If it isn’t balanced, it is like a recipe that doesn’t include any amounts. It would be useless to try to make something from such a recipe. ...
Frequency, temperature and salinity variation of the
... model is based on the assumption that there are no intermolecular interactions and this simple model does not accurately predict the permittivity of real dielectrics. Several dielectrics may be better modelled using the Cole-Cole model [5] which states that the relative permittivity is given by ...
... model is based on the assumption that there are no intermolecular interactions and this simple model does not accurately predict the permittivity of real dielectrics. Several dielectrics may be better modelled using the Cole-Cole model [5] which states that the relative permittivity is given by ...
Chapter 4: Aqueous Solutions (Chs 4 and 5 in Jespersen, Ch4 in
... 2) All the atoms of the free element have oxidation states of zero. 3) Metals in groups 1A have oxidation states of +1, 2A are +2, and Al is +3. 4) In compounds, H has oxidation state of +1, F has –1. 5) Oxygen has –2 oxidation number. 6) Group 7A elements have –1 oxidation numbers. 7) Group 6A elem ...
... 2) All the atoms of the free element have oxidation states of zero. 3) Metals in groups 1A have oxidation states of +1, 2A are +2, and Al is +3. 4) In compounds, H has oxidation state of +1, F has –1. 5) Oxygen has –2 oxidation number. 6) Group 7A elements have –1 oxidation numbers. 7) Group 6A elem ...
Theory of Ion Exchange
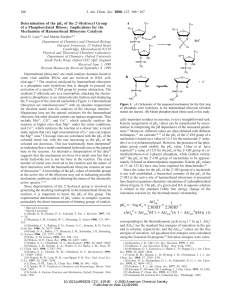
... dependence of kd on CA (log kd"log (kA/BQ)!log CB) is that CA CB/kA/B. Thus, even if CA CB, the dependence may not be linear if the selectivity coefRcient is very large. This feature of kd is shown as calculated examples in Figure 2. It can be seen that, if the selectivity coefRcient is low, kd fall ...
... dependence of kd on CA (log kd"log (kA/BQ)!log CB) is that CA CB/kA/B. Thus, even if CA CB, the dependence may not be linear if the selectivity coefRcient is very large. This feature of kd is shown as calculated examples in Figure 2. It can be seen that, if the selectivity coefRcient is low, kd fall ...
The Free High School Science Texts: A Textbook for High School
... Some years later, it was discovered (by Rutherford in 1911) that atoms have a positively charged nucleus (centre) with the negative electrons moving around it. This proved that the plum pudding model was wrong and scientists then pictured the atom like a mini solar system where the electrons orbit t ...
... Some years later, it was discovered (by Rutherford in 1911) that atoms have a positively charged nucleus (centre) with the negative electrons moving around it. This proved that the plum pudding model was wrong and scientists then pictured the atom like a mini solar system where the electrons orbit t ...
Batteries are all over the place -- in our cars, our
... wire. Some of the heat energy is turned into electron motion. The electrons go to the trouble to move to the carbon rod because they find it easier to combine with hydrogen there. There is a characteristic voltage in the cell of 0.76 volts. Eventually, the zinc rod dissolves completely or the hydrog ...
... wire. Some of the heat energy is turned into electron motion. The electrons go to the trouble to move to the carbon rod because they find it easier to combine with hydrogen there. There is a characteristic voltage in the cell of 0.76 volts. Eventually, the zinc rod dissolves completely or the hydrog ...
LEGGETT--AP CHEMISTRY * MINIMAL FINAL REVIEW
... bonds in a molecule? (ignore any subsequent bond formation that may occur) A. Always exothermic B. Always endothermic C. Net energy change is zero D. Exothermic or endothermic depending on conditions. 17. How many sigma (σ) and pi(π) electron pairs are there in a carbon dioxide molecule? A. Two sigm ...
... bonds in a molecule? (ignore any subsequent bond formation that may occur) A. Always exothermic B. Always endothermic C. Net energy change is zero D. Exothermic or endothermic depending on conditions. 17. How many sigma (σ) and pi(π) electron pairs are there in a carbon dioxide molecule? A. Two sigm ...
PHYSICAL SETTING CHEMISTRY
... 57 Describe, in terms of valence electrons, how the chemical bonds form in the substance represented in diagram 1. [1] 58 Determine the total number of electrons in the bonds between the nitrogen atom and the three hydrogen atoms represented in diagram 2. [1] 59 Explain, in terms of distribution of ...
... 57 Describe, in terms of valence electrons, how the chemical bonds form in the substance represented in diagram 1. [1] 58 Determine the total number of electrons in the bonds between the nitrogen atom and the three hydrogen atoms represented in diagram 2. [1] 59 Explain, in terms of distribution of ...
13.0 Redox Reactions PowerPoint
... Notice that both of these half-reactions are balanced by mass (same number of atoms/ions of each element on both sides) and by charge (same total charge on both sides) ...
... Notice that both of these half-reactions are balanced by mass (same number of atoms/ions of each element on both sides) and by charge (same total charge on both sides) ...
Laboratory 3
... In this equation, the (+) symbol indicates that nitrogen reacts with oxygen and the arrow indicates that nitric oxide is formed. The chemical formulas on the left side of the equation are collectively known as the reactants and those on the right side as the products. In this case we have one kind o ...
... In this equation, the (+) symbol indicates that nitrogen reacts with oxygen and the arrow indicates that nitric oxide is formed. The chemical formulas on the left side of the equation are collectively known as the reactants and those on the right side as the products. In this case we have one kind o ...
3 center 4 electron bond article
... can be extended to the study of bonding in SF6 and PF6–. Unlike the pentacoordinated AB5-type molecules (D3h symmetry), in the hexacoordinated AB6-type molecules such as SF6 and PF6– (Oh symmetry), the six fluorine ligands approach to the central sulfur or phosphorus atom along the x, y, and z axes ...
... can be extended to the study of bonding in SF6 and PF6–. Unlike the pentacoordinated AB5-type molecules (D3h symmetry), in the hexacoordinated AB6-type molecules such as SF6 and PF6– (Oh symmetry), the six fluorine ligands approach to the central sulfur or phosphorus atom along the x, y, and z axes ...
and Lead Bis(tri-tert-butoxystannate)
... related and similar coordination spheres of Si", Ge" and Sn" with ligands such as C(PMe,)F cf. Ref. [6]). The unusually long P b F e contacts in 3 are unmistakable when compared with the Pb-Fe distances in [Pb{Fe(CO),},] (2.62(1) A)[71 or in the dianion [Pb{Fe(CO),),Fe,(C0),]20 (2.62(1)2.83(1) A).[* ...
... related and similar coordination spheres of Si", Ge" and Sn" with ligands such as C(PMe,)F cf. Ref. [6]). The unusually long P b F e contacts in 3 are unmistakable when compared with the Pb-Fe distances in [Pb{Fe(CO),},] (2.62(1) A)[71 or in the dianion [Pb{Fe(CO),),Fe,(C0),]20 (2.62(1)2.83(1) A).[* ...
Chapter 8 Concepts of Chemical Bonding
... lattice energy is correct for these ionic compounds. (a) NaCl > MgO > CsI > ScN, (b) ScN > MgO > NaCl > CsI, (c) NaCl > CsI > ScN > CaO, (d) MgO > NaCl > ScN > CsI, (e) ScN > CsI > NaCl > MgO. ...
... lattice energy is correct for these ionic compounds. (a) NaCl > MgO > CsI > ScN, (b) ScN > MgO > NaCl > CsI, (c) NaCl > CsI > ScN > CaO, (d) MgO > NaCl > ScN > CsI, (e) ScN > CsI > NaCl > MgO. ...
Hydroxyl Group of a Phosphorylated Ribose
... of several metal ions (e.g., Pb2+(7.7), Zn2+(9.0), and Mg2+(11.4)32) that readily catalyze the reaction. It therefore seems unlikely that metal hydroxide ions act as the general base in the reaction.10 Since a metal ion is known to bind directly to the adjacent pro-R oxygen of the phosphate, it is c ...
... of several metal ions (e.g., Pb2+(7.7), Zn2+(9.0), and Mg2+(11.4)32) that readily catalyze the reaction. It therefore seems unlikely that metal hydroxide ions act as the general base in the reaction.10 Since a metal ion is known to bind directly to the adjacent pro-R oxygen of the phosphate, it is c ...
Activation parameters for ET
... Due to entropy mixing, the slope of the pH-dependence should be negative. Observed (total) activation entropy change The slope of the pH-dependence of the observed (total) entropy change is positive (or in some cases slightly negative) Our data indicate that the magnitude of ΔGET is at least as larg ...
... Due to entropy mixing, the slope of the pH-dependence should be negative. Observed (total) activation entropy change The slope of the pH-dependence of the observed (total) entropy change is positive (or in some cases slightly negative) Our data indicate that the magnitude of ΔGET is at least as larg ...