A quantum mechanically guided view of Cd-MOF-5 from
... ity and to establish equilibrium structural parameters. The generalized gradient approximation (GGA) [45–47] includes the effects of local gradients in the charge density for each point in the materials and generally gives better equilibrium structural parameters than the local density approximation ...
... ity and to establish equilibrium structural parameters. The generalized gradient approximation (GGA) [45–47] includes the effects of local gradients in the charge density for each point in the materials and generally gives better equilibrium structural parameters than the local density approximation ...
Chapter 5 CHEM 121
... that are dissolved in water. Ionic compounds and some polar covalent compounds break apart (dissociate) when they dissolve in water and form ions. ...
... that are dissolved in water. Ionic compounds and some polar covalent compounds break apart (dissociate) when they dissolve in water and form ions. ...
Practice Exam II
... = 2. Thus there are two empirical formulas in a molecular formula. Therefore, the molecular formula is C2H6O2 18. If the equation C4H10 + O2 CO2 + H2O is balanced, which of the following quantities is correct? Note: Coefficients must be integers. A) 2 C4H10, 13 O2, 8 CO2, and 10 H2O B) 13 C4H10, 2 ...
... = 2. Thus there are two empirical formulas in a molecular formula. Therefore, the molecular formula is C2H6O2 18. If the equation C4H10 + O2 CO2 + H2O is balanced, which of the following quantities is correct? Note: Coefficients must be integers. A) 2 C4H10, 13 O2, 8 CO2, and 10 H2O B) 13 C4H10, 2 ...
Chap 3 - HCC Learning Web
... = 2. Thus there are two empirical formulas in a molecular formula. Therefore, the molecular formula is C2H6O2 18. If the equation C4H10 + O2 CO2 + H2O is balanced, which of the following quantities is correct? Note: Coefficients must be integers. A) 2 C4H10, 13 O2, 8 CO2, and 10 H2O B) 13 C4H10, 2 ...
... = 2. Thus there are two empirical formulas in a molecular formula. Therefore, the molecular formula is C2H6O2 18. If the equation C4H10 + O2 CO2 + H2O is balanced, which of the following quantities is correct? Note: Coefficients must be integers. A) 2 C4H10, 13 O2, 8 CO2, and 10 H2O B) 13 C4H10, 2 ...
Net ionic equation
... because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive or negative charge. The reason these partial charges exist will be discussed later in the semester. Because cations and ani ...
... because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive or negative charge. The reason these partial charges exist will be discussed later in the semester. Because cations and ani ...
Chemical Reactions: Helpful Hints
... (Ag+ in the 1+ oxidation state). Did you observe a band of shiny metal being formed at the interface of the solid and the solution (Hmm, what could that be? What was in solution that would give such luster?) Reaction 11 involves two metals that reacted to become ions in aqueous solution (i.e. both g ...
... (Ag+ in the 1+ oxidation state). Did you observe a band of shiny metal being formed at the interface of the solid and the solution (Hmm, what could that be? What was in solution that would give such luster?) Reaction 11 involves two metals that reacted to become ions in aqueous solution (i.e. both g ...
VUV photochemistry of small biomolecules
... are very important processes in the VUV, mass spectrometry is an excellent tool to get more insight into VUV photochemistry. In our experiments, monochromatised SR is focused into a differentially pumped gas cell which can be heated up to 400 1C in order to vaporise compounds in the liquid or solid ...
... are very important processes in the VUV, mass spectrometry is an excellent tool to get more insight into VUV photochemistry. In our experiments, monochromatised SR is focused into a differentially pumped gas cell which can be heated up to 400 1C in order to vaporise compounds in the liquid or solid ...
North Carolina Test of Physical Science
... written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
... written permission from the North Carolina Department of Public Instruction, Raleigh, North Carolina. ...
ism ismismismismismrapidrevisionquestionsismismismismismism
... State a feature to distinguish (i) Metallic solid from ionic solid (ii) Covalent solid from molecular solid Constituent particles in metallic solids are positive metal ions (kernels) and mobile electrons. Constituent particles in ionic solids are positive ions and negative ions. Constituent particle ...
... State a feature to distinguish (i) Metallic solid from ionic solid (ii) Covalent solid from molecular solid Constituent particles in metallic solids are positive metal ions (kernels) and mobile electrons. Constituent particles in ionic solids are positive ions and negative ions. Constituent particle ...
Ex: -F, -Cl, -Br
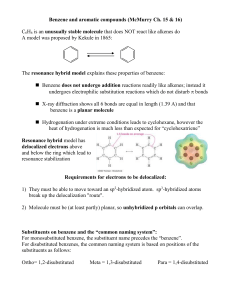
... 2) Continuous overlap of unhybridized p-orbitals forming a delocalized -cloud of e3) The number of -electrons must be equal to 4n + 2 (where n = 0, 1, 2…) Huckel’s Rule (4n + 2 rule) relates to the way electrons populate MOs: Aromatic compounds: molecules with 4n+2 e- have just enough to pop ...
... 2) Continuous overlap of unhybridized p-orbitals forming a delocalized -cloud of e3) The number of -electrons must be equal to 4n + 2 (where n = 0, 1, 2…) Huckel’s Rule (4n + 2 rule) relates to the way electrons populate MOs: Aromatic compounds: molecules with 4n+2 e- have just enough to pop ...
Module 4 : Organoelement compounds of Group 15 Lecture 1
... Due to the strong oxidizing nature of pentahalides, the direct alkylation or arylation to generate ER5 is not feasible, but can be prepared in two steps. A few representative methods of preparation are given below: ...
... Due to the strong oxidizing nature of pentahalides, the direct alkylation or arylation to generate ER5 is not feasible, but can be prepared in two steps. A few representative methods of preparation are given below: ...
Chem 173: Final Exam Review Short Answer and Problems 1
... The reaction 2 A + B Ÿ C obeys the following rate equation: Rate = k[A][B]1/2. The order of the reaction with respect to B is ___________________, and the total order of the reaction is ____________________. The units of k are _____________________. If the rate of formation of C is 2.00 mol•L—1•s—1, ...
... The reaction 2 A + B Ÿ C obeys the following rate equation: Rate = k[A][B]1/2. The order of the reaction with respect to B is ___________________, and the total order of the reaction is ____________________. The units of k are _____________________. If the rate of formation of C is 2.00 mol•L—1•s—1, ...
ELECTROLYTE CONDUCTANCE
... Example 3 For NH4OH electrolyte: v+ = 1 and v- = 1 Since 1NH4+ ion present for each OH- ion present in solution. Example 4 For K4Fe(CN)6 electrolyte: v+ = 4 and v- = 1 Since there are 4K+ ions present for each Fe(CN)4-6 ion present in solution. ...
... Example 3 For NH4OH electrolyte: v+ = 1 and v- = 1 Since 1NH4+ ion present for each OH- ion present in solution. Example 4 For K4Fe(CN)6 electrolyte: v+ = 4 and v- = 1 Since there are 4K+ ions present for each Fe(CN)4-6 ion present in solution. ...
Practice Exam II
... signs and put them as subscripts; be careful that since phosphate has subscript in oxygen atom and to avoid confusion, we must write is as Mn3(PO4)2 instead of Mn3PO42. Because the former indicates there are two P atoms and 2x4=8 oxygen atoms, while the latter indicates there is one P atom and ther ...
... signs and put them as subscripts; be careful that since phosphate has subscript in oxygen atom and to avoid confusion, we must write is as Mn3(PO4)2 instead of Mn3PO42. Because the former indicates there are two P atoms and 2x4=8 oxygen atoms, while the latter indicates there is one P atom and ther ...
VSEPR Model
... nitrogen atom in the ammonia molecule. (b) Three of the electron pairs around nitrogen are shared with hydrogen atoms as shown and one is a lone pair. Although the arrangement of electron pairs is tetrahedral, as in the methane molecule, the hydrogen atoms in the ammonia molecule occupy only three c ...
... nitrogen atom in the ammonia molecule. (b) Three of the electron pairs around nitrogen are shared with hydrogen atoms as shown and one is a lone pair. Although the arrangement of electron pairs is tetrahedral, as in the methane molecule, the hydrogen atoms in the ammonia molecule occupy only three c ...
Enthalpy Change of Hydrogen Bond Formation between
... The data for p-cresol indicate that the tertiary amines are essentially identical in enthalpy change while the primary amine has a higher enthalpy change. Two of these values are similar to values obtained by UV spectrophotometry3 and follow the same pattern as the equilibrium constants for the same ...
... The data for p-cresol indicate that the tertiary amines are essentially identical in enthalpy change while the primary amine has a higher enthalpy change. Two of these values are similar to values obtained by UV spectrophotometry3 and follow the same pattern as the equilibrium constants for the same ...
LA Mixtures, chemical elements and bingo
... Our next class deals with simple concepts of chemistry. Most of it is a review of concepts already studied but we would like the students to pay attention to the English pronunciation. These are the activities: 1. Write on the board: “Pure substances and mixtures”. Remind them the meaning of these w ...
... Our next class deals with simple concepts of chemistry. Most of it is a review of concepts already studied but we would like the students to pay attention to the English pronunciation. These are the activities: 1. Write on the board: “Pure substances and mixtures”. Remind them the meaning of these w ...
EXAM 3
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
... The elements nitrogen and oxygen combine at high temperatures to form nitric oxide, NO. The balanced chemical equation is N2(g) + O2(g) ----------> 2NO(g) In a high temperature experiment, a chemist mixed 3.417 g of N2 with an excess of O2 and allowed the above reaction to take place. Assuming compl ...
Chemistry 14C Winter 2017 Final Exam Part A Solutions Page 1
... 20. 57 oxygens. Lipids are amphiphilic or nonpolar, so less oxygen atoms are more likely than more oxygen atoms. 21. (a) LiF is the only ionic answer choice. (b) FI has the most polar bonds and the larges London forces. (c) HOCH2CH2OH has the most hydrogen bond donors and the most hydrogen bond acce ...
... 20. 57 oxygens. Lipids are amphiphilic or nonpolar, so less oxygen atoms are more likely than more oxygen atoms. 21. (a) LiF is the only ionic answer choice. (b) FI has the most polar bonds and the larges London forces. (c) HOCH2CH2OH has the most hydrogen bond donors and the most hydrogen bond acce ...
- Te Kura
... and neutrons. 2. Protons are positively charged particles held tightly together in a very small space in the centre of the atom, called the nucleus. 3. If more than one proton is present, the atom needs neutrons to stop the protons repelling each other and flying apart. These neutral particles are ...
... and neutrons. 2. Protons are positively charged particles held tightly together in a very small space in the centre of the atom, called the nucleus. 3. If more than one proton is present, the atom needs neutrons to stop the protons repelling each other and flying apart. These neutral particles are ...
precipitation rxn_level_packet
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
QualGroupB - Back To Home Page
... is helpful in this analysis since doing so will allow you to observe what a positive test looks like. It is usually convenient to test a known sample simultaneously with your unknown. To test a known sample, you can either prepare a known mixture of the Group B cations or the known Group B cation so ...
... is helpful in this analysis since doing so will allow you to observe what a positive test looks like. It is usually convenient to test a known sample simultaneously with your unknown. To test a known sample, you can either prepare a known mixture of the Group B cations or the known Group B cation so ...
The Major Classes of Chemical Reactions
... (d) 35 mL of 0.84 M zinc chloride Plan We write an equation that shows 1 mol of compound dissociating into ions. In (a), we multiply the moles of ions by 5.0. In (b), we first convert grams to moles. In (c), we first convert formula units to moles. In (d), we first convert molarity and volume to moles. ...
... (d) 35 mL of 0.84 M zinc chloride Plan We write an equation that shows 1 mol of compound dissociating into ions. In (a), we multiply the moles of ions by 5.0. In (b), we first convert grams to moles. In (c), we first convert formula units to moles. In (d), we first convert molarity and volume to moles. ...