Textbook Answer Keys - Mr. Massey`s Chemistry Pages
... The Bohr theory provided a first approximation of atomic structure, and in particular the arrangement of electrons; it has since been replaced by more sophisticated mathematical theories from the field of quantum mechanics, which incorporate the wave-like nature of the electron; the wavefunctions of ...
... The Bohr theory provided a first approximation of atomic structure, and in particular the arrangement of electrons; it has since been replaced by more sophisticated mathematical theories from the field of quantum mechanics, which incorporate the wave-like nature of the electron; the wavefunctions of ...
IV. The Transmission Electron Microscope
... The sputter ion pump works by ionizing gas that falls within a magnetically confined cold cathode, then reacting these with (or burying them in) plates of titanium. The ion pump consists of two parallel plates (3) of negatively-charged titanium (cathode) and cylindrical anodes (4) all surrounded by ...
... The sputter ion pump works by ionizing gas that falls within a magnetically confined cold cathode, then reacting these with (or burying them in) plates of titanium. The ion pump consists of two parallel plates (3) of negatively-charged titanium (cathode) and cylindrical anodes (4) all surrounded by ...
chemistry advanced may 2010 marking scheme
... The oxidation of NO is slow and air is blown into the nitrous gas which is passed up through baffles against a flow of dilute nitric acid in a large tower in which oxidation and absorption is completed. (0.5 marks) NB The Ostwald Process (above) has displaced the obsolete Birkeland-Eyde Process invo ...
... The oxidation of NO is slow and air is blown into the nitrous gas which is passed up through baffles against a flow of dilute nitric acid in a large tower in which oxidation and absorption is completed. (0.5 marks) NB The Ostwald Process (above) has displaced the obsolete Birkeland-Eyde Process invo ...
A STUDY ON STRUCTURAL ASPECTS AND MICROBIAL ACTIVITY OF (E)-4- PYRIDINECARBOXALDEHYDE-3-HYDROXY-5-(HYDROXYMETHYL)-2-METHYL-OXIME
... was done against F2 using SHELXL-97[15]. All non-hydrogen atoms were refined anisotropically and hydrogens were introduced on calculated positions and included in the refinement riding on their respective parent atoms. The pH measurements were made using a digital ELICO electronic model LI 120 pH me ...
... was done against F2 using SHELXL-97[15]. All non-hydrogen atoms were refined anisotropically and hydrogens were introduced on calculated positions and included in the refinement riding on their respective parent atoms. The pH measurements were made using a digital ELICO electronic model LI 120 pH me ...
KHOA: HÓA HỌC - CCS - Trường Đại học Sư phạm Hà Nội
... element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into simpler substances by chemical methods. Currently, there are about 118 ...
... element is a substance comprised of a single type of atom. The elements are the building blocks of our nature. An element is either discovered in nature or synthesized in the laboratory in pure form that cannot be separated into simpler substances by chemical methods. Currently, there are about 118 ...
Final Review 2006
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
CHEM102 Chemistry II Spring 11-12 Mid
... B) there are equal numbers of molecules on each side of the reaction arrow. C) there are equal numbers of atoms on each side of the reaction arrow. D) the number of atoms depends present in a reaction can vary when the conditions change during the reaction. E) none of the above 4) The oxidation numb ...
... B) there are equal numbers of molecules on each side of the reaction arrow. C) there are equal numbers of atoms on each side of the reaction arrow. D) the number of atoms depends present in a reaction can vary when the conditions change during the reaction. E) none of the above 4) The oxidation numb ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... • brass, a mixture of z).................... and ................... • aa)...................................., with a very low melting point, is an alloy of ab).................................. and .................................... and is used in ac)..................................... and ... ...
... • brass, a mixture of z).................... and ................... • aa)...................................., with a very low melting point, is an alloy of ab).................................. and .................................... and is used in ac)..................................... and ... ...
Atoms and Molecules - New Age International
... The above expression is known as uncertainty relation where ∆ x = change in position, ∆p = change in momentum and h = Plancks constant. The relation implies that a simultaneous and precise measurement of both position and momentum (velocity) of a dynamic particle like electron is impossible and the ...
... The above expression is known as uncertainty relation where ∆ x = change in position, ∆p = change in momentum and h = Plancks constant. The relation implies that a simultaneous and precise measurement of both position and momentum (velocity) of a dynamic particle like electron is impossible and the ...
SUP1111 11 - The Open University
... element. The total number of protons and neutrons (which together are called ______________) in an atom gives its ____________. A _____________ is created by the ___________ of one or more electrons from a neutral atom. If an atom should ______________ one or more electrons it becomes a ____________ ...
... element. The total number of protons and neutrons (which together are called ______________) in an atom gives its ____________. A _____________ is created by the ___________ of one or more electrons from a neutral atom. If an atom should ______________ one or more electrons it becomes a ____________ ...
UNIT 2 ATOMS, MATTER, AND THE MOLE
... there are two atoms of hydrogen. 2. H2O2 is not water. It is called hydrogen peroxide, has two atoms of hydrogen for every two atoms of oxygen and behaves much differently that water. This brings us to the next law. F. LAW OF MULTIPLE PROPORTIONS-states that there can exist two or more compounds wit ...
... there are two atoms of hydrogen. 2. H2O2 is not water. It is called hydrogen peroxide, has two atoms of hydrogen for every two atoms of oxygen and behaves much differently that water. This brings us to the next law. F. LAW OF MULTIPLE PROPORTIONS-states that there can exist two or more compounds wit ...
Protons, neutrons and electrons Isotopes Atomic mass units and
... H atom, but that would be too obvious! Chemists use one-twelfth the mass of a 12C atom instead, which if you think about it, amounts to the same thing. As there are six protons and six neutrons in this nucleus, dividing its mass by 12 gives you the mass of a proton or neutron. However, it really is ...
... H atom, but that would be too obvious! Chemists use one-twelfth the mass of a 12C atom instead, which if you think about it, amounts to the same thing. As there are six protons and six neutrons in this nucleus, dividing its mass by 12 gives you the mass of a proton or neutron. However, it really is ...
Students should be able to - Dover College Science
... a solution of sodium or potassium cyanide in water with sulphuric acid. The pH of the solution is adjusted to about 4 - 5, because this gives the fastest reaction. The reaction happens at room temperature. The solution will contain hydrogen cyanide (from the reaction between the sodium or potassium ...
... a solution of sodium or potassium cyanide in water with sulphuric acid. The pH of the solution is adjusted to about 4 - 5, because this gives the fastest reaction. The reaction happens at room temperature. The solution will contain hydrogen cyanide (from the reaction between the sodium or potassium ...
Mass # = Atomic # + # Neutrons
... Atomic number is the number of protons in the nucleus of an element. Since in neutral atoms the number of protons is equal to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For e ...
... Atomic number is the number of protons in the nucleus of an element. Since in neutral atoms the number of protons is equal to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For e ...
1. formulae equations and amount
... Heat filtrate solution until volume reduced by half Leave solution to cool and allow remaining water to evaporate Slowly and crystals to form Filter or pick out crystals Leave to dry and put crystals between filter ...
... Heat filtrate solution until volume reduced by half Leave solution to cool and allow remaining water to evaporate Slowly and crystals to form Filter or pick out crystals Leave to dry and put crystals between filter ...
unit 7 h chem notes - chemical equations
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
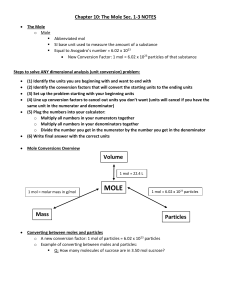
MOLE Mass Volume Particles
... o Mass in grams of one mole of any pure substance o Equal to the atomic mass of an element o Found on Periodic Table in amu o Units are g/mol Converting between moles and mass o Q: How many grams of Cu are in 3.00 mol of Cu? ...
... o Mass in grams of one mole of any pure substance o Equal to the atomic mass of an element o Found on Periodic Table in amu o Units are g/mol Converting between moles and mass o Q: How many grams of Cu are in 3.00 mol of Cu? ...
Formation and Stability of High-Spin Alkali Clusters - Max-Born
... difference in the selection of spin-polarized clusters [33]. On the other hand, the binding of atoms and molecules towards the surface of a helium droplet slightly increases from 13.2 cm−1 to 15.6 cm−1 going from sodium to cesium [15]. Taking into account the substantial increase in the mass of the ...
... difference in the selection of spin-polarized clusters [33]. On the other hand, the binding of atoms and molecules towards the surface of a helium droplet slightly increases from 13.2 cm−1 to 15.6 cm−1 going from sodium to cesium [15]. Taking into account the substantial increase in the mass of the ...
The Diagnostics System at the Cryogenic Storage Ring CSR
... beam current for any ion species having different ion velocities. Because the spectrum of the pick-up signal scales with the inverse of the ion velocity (compare equation 4) this method to determine the absolute intensity of an electron cooled, bunched ion beam is very sensitive for low velocity ion ...
... beam current for any ion species having different ion velocities. Because the spectrum of the pick-up signal scales with the inverse of the ion velocity (compare equation 4) this method to determine the absolute intensity of an electron cooled, bunched ion beam is very sensitive for low velocity ion ...
GROUP 13 ELEMENTS -THE BORON FAMILY -
... associated with increasing size. However, B and Al follows immediately after s block elements, while Ga, In and Tl follows after d block elements. So the extra d-electrons in Ga, In and Tl do not shield the nuclear very effectively, so that the orbital electrons are more firmly or tightly held and t ...
... associated with increasing size. However, B and Al follows immediately after s block elements, while Ga, In and Tl follows after d block elements. So the extra d-electrons in Ga, In and Tl do not shield the nuclear very effectively, so that the orbital electrons are more firmly or tightly held and t ...
down
... If box length ↑, kinetic energy ↓. Therefore, if the electron is delocalized over the whole molecule, kinetic energy decrease. → bond formation? MS310 Quantum Physical Chemistry ...
... If box length ↑, kinetic energy ↓. Therefore, if the electron is delocalized over the whole molecule, kinetic energy decrease. → bond formation? MS310 Quantum Physical Chemistry ...
Chemical Reactions
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Analyze: We are given several chemical formulas and asked to classify each substance as a strong electrolyte, weak electrolyte, or nonelectrolyte. Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Sec ...
... Analyze: We are given several chemical formulas and asked to classify each substance as a strong electrolyte, weak electrolyte, or nonelectrolyte. Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Sec ...
MERIDIAN PUBLIC SCHOOL DISTRICT
... Schrődinger and describe how each discovery contributed to the current model of atomic and nuclear structure. (DOK 2) dWrite appropriate equations for nuclear decay reactions, describe how the nucleus changes during these reactions, and compare the resulting radiation with regard to penetrating abil ...
... Schrődinger and describe how each discovery contributed to the current model of atomic and nuclear structure. (DOK 2) dWrite appropriate equations for nuclear decay reactions, describe how the nucleus changes during these reactions, and compare the resulting radiation with regard to penetrating abil ...