Chem13-14PrecipABNeut
... with extensive memory during tests. If a type of calculator is specified for your course, buy two if possible. When one becomes broken or lost, you will have a familiar backup if the bookstore is sold out later in the term. If no type of calculator is specified for your course, any inexpensive calcu ...
... with extensive memory during tests. If a type of calculator is specified for your course, buy two if possible. When one becomes broken or lost, you will have a familiar backup if the bookstore is sold out later in the term. If no type of calculator is specified for your course, any inexpensive calcu ...
Slide 1
... I. Oxidation & Reduction -_________ oxidation is defined as the ____ loss of ________ _____ of a ________ electrons from the atoms substance -during its reaction with Chlorine _______, Sodium ______ _____ loses ________: electrons oxidation number is equal to the number of electrons lost or gained b ...
... I. Oxidation & Reduction -_________ oxidation is defined as the ____ loss of ________ _____ of a ________ electrons from the atoms substance -during its reaction with Chlorine _______, Sodium ______ _____ loses ________: electrons oxidation number is equal to the number of electrons lost or gained b ...
Chemical Bonding II
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
Mnemonic Devices - Free WonderKids-e
... III) Oxidation – Reduction Reaction for Metal with Non-Metal – driven by Electron Transfer (and possibly Formation of Gas), (Electron transfer reactions can also take place between two non-metals, and the compound formed is not ionic. A sure sign of that happening is the presence of oxygen, O 2 ( g ...
... III) Oxidation – Reduction Reaction for Metal with Non-Metal – driven by Electron Transfer (and possibly Formation of Gas), (Electron transfer reactions can also take place between two non-metals, and the compound formed is not ionic. A sure sign of that happening is the presence of oxygen, O 2 ( g ...
ION SELECTIVE ELECTRODES
... • An electrode that responds to a particular ion’s activity is called ion-selective (or) ion-sensitive electrode (ISE). • Principle: When ever two solutions of different concentrations are separated by a membrane, a potential difference is set up across the membrane due to the unequal distribution o ...
... • An electrode that responds to a particular ion’s activity is called ion-selective (or) ion-sensitive electrode (ISE). • Principle: When ever two solutions of different concentrations are separated by a membrane, a potential difference is set up across the membrane due to the unequal distribution o ...
Chemistry - Chap 12 Homework Answers 2014
... small amount of liquid? What processes are going on in the flask? pressure exerted by vapor above a liquid. High energy particles at surface escape and exert the pressure 8. Which substance in each pair would be expected to show the largest vapor pressure at a given temperature? The largest vapor pr ...
... small amount of liquid? What processes are going on in the flask? pressure exerted by vapor above a liquid. High energy particles at surface escape and exert the pressure 8. Which substance in each pair would be expected to show the largest vapor pressure at a given temperature? The largest vapor pr ...
Energetics - chemistryatdulwich
... The lattice energy is an also indicator of the stability of an ionic compound: the larger the value the more stable the compound, the more tightly held are the ions within the lattice. Lattice enthalpy cannot be measured directly but can be calculated using a Born-Haber cycle which is an energy cycl ...
... The lattice energy is an also indicator of the stability of an ionic compound: the larger the value the more stable the compound, the more tightly held are the ions within the lattice. Lattice enthalpy cannot be measured directly but can be calculated using a Born-Haber cycle which is an energy cycl ...
Enthalpy change - Don`t Trust Atoms
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
... • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants ...
Version 1.6 - Clark Science Center
... r1,2 is the distance separating them, and the first term is just there to convert from units of charge, Coulombs, and distance, to energy units. Note when both the charges are the same, either positive or negative, then the potential energy is more positive, less stable, when the distance is small. ...
... r1,2 is the distance separating them, and the first term is just there to convert from units of charge, Coulombs, and distance, to energy units. Note when both the charges are the same, either positive or negative, then the potential energy is more positive, less stable, when the distance is small. ...
UNIVERSITI MALAYSIA SABAH
... Because of the presence of a lone pair of electron on nitrogen atom in ammonia, it forms a number of complexes with cations involving dative or coordinate bond. It acts as a monodentate ligand. When ammonia is added to an aqueous solution containing copper(II) ions, a deep blue complex cation [Cu(NH ...
... Because of the presence of a lone pair of electron on nitrogen atom in ammonia, it forms a number of complexes with cations involving dative or coordinate bond. It acts as a monodentate ligand. When ammonia is added to an aqueous solution containing copper(II) ions, a deep blue complex cation [Cu(NH ...
chemistry writing team
... (d) cosmic rays from outer space (e) X-rays [Ans. cosmic rays < X-rays < amber light < microwaves < FM] 9. Which of the following will not show deflection from the path on passing through an electric field Proton, Cathode rays, Anode rays, Electron, Neutron. [Hint : Neutron (n) will not show deflect ...
... (d) cosmic rays from outer space (e) X-rays [Ans. cosmic rays < X-rays < amber light < microwaves < FM] 9. Which of the following will not show deflection from the path on passing through an electric field Proton, Cathode rays, Anode rays, Electron, Neutron. [Hint : Neutron (n) will not show deflect ...
universita` degli studi di padova - Dipartimento di Scienze Chimiche
... (b) – For higher electrolyte conc. A significant secondary minimum appears before the energy barrier. The potential energy minimum at constant is called primary minimum. The colloids are kinetically stable because overcoming the energy barrier is a slow process and the particles either sit in the se ...
... (b) – For higher electrolyte conc. A significant secondary minimum appears before the energy barrier. The potential energy minimum at constant is called primary minimum. The colloids are kinetically stable because overcoming the energy barrier is a slow process and the particles either sit in the se ...
Laser and its applications
... of laser medium used (e.g. He-Ne, CO2 and Nd:YAG). This laser medium may be gas, liquid, or solid, determines the wavelength of the laser radiation. In some lasers the amplifying medium consists of two parts, the laser host medium and the laser atoms. For example, in Nd: YAG laser, the host medium i ...
... of laser medium used (e.g. He-Ne, CO2 and Nd:YAG). This laser medium may be gas, liquid, or solid, determines the wavelength of the laser radiation. In some lasers the amplifying medium consists of two parts, the laser host medium and the laser atoms. For example, in Nd: YAG laser, the host medium i ...
Packet 1 - Kentucky Community and Technical College System
... Step 1 Write the reactants as they actually exist before any reaction occurs (the complete ionic equation). Remember that when a salt dissolves, its ions completely separate. Step 2 Consider the various solids that could form. To do this, simply exchange the anions (or the cations) of the added ...
... Step 1 Write the reactants as they actually exist before any reaction occurs (the complete ionic equation). Remember that when a salt dissolves, its ions completely separate. Step 2 Consider the various solids that could form. To do this, simply exchange the anions (or the cations) of the added ...
Chemistry notes Important terms *Mass of element in a sample
... Polar molecule : a molecule that is electronegativity charged Precipitate reaction- when two soluble ionic compounds combined to form an insoluble product Acid-base reaction- acid reacts with a base to form a neutral substance Titration- one solution of known concentration is used to determine the c ...
... Polar molecule : a molecule that is electronegativity charged Precipitate reaction- when two soluble ionic compounds combined to form an insoluble product Acid-base reaction- acid reacts with a base to form a neutral substance Titration- one solution of known concentration is used to determine the c ...
Final Study Guide (Semester 2) Answer Key
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
... ***The first thing you should do when solving this is look at the common ion chart and write down all the ions. It’s much easier than looking them up again for each question. a. Write the balanced molecular equation. Include phase symbols. Ba(NO3)2(aq) + K2SO4(aq) BaSO4(s) + 2KNO3 (aq) Switch the c ...
Introduction to Organic Chemistry 2 ed William H. Brown
... These contributing structures are equivalent; the carboxylate anion is stabilized by delocalization of the negative charge Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved. ...
... These contributing structures are equivalent; the carboxylate anion is stabilized by delocalization of the negative charge Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved. ...
Unit 2:
... 2. Suppose that a stable element with atomic number 119, symbol Q, has been discovered. (a) Write the ground-state electron configuration for Q, showing only the valence-shell electrons. (b) Would Q be a metal or a nonmetal? Explain in terms of electron configuration. (c) On the basis of periodic tr ...
... 2. Suppose that a stable element with atomic number 119, symbol Q, has been discovered. (a) Write the ground-state electron configuration for Q, showing only the valence-shell electrons. (b) Would Q be a metal or a nonmetal? Explain in terms of electron configuration. (c) On the basis of periodic tr ...
Chapter 4: Experimental Techniques
... the sample and of a series of standards. The standards contain known concentrations of the metal being analysed and are used to construct a calibration curve (see below). The atomic absorption spectrometer (Fig. 4.6) contains either a flame atomizer, a graphite furnace or an electrically heated atom ...
... the sample and of a series of standards. The standards contain known concentrations of the metal being analysed and are used to construct a calibration curve (see below). The atomic absorption spectrometer (Fig. 4.6) contains either a flame atomizer, a graphite furnace or an electrically heated atom ...
Ionization Potential and Structure Relaxation of Adenine, Thymine
... biological systems. Pairing is also the mechanism by which codons on messenger RNA molecules are recognized by anticodons on transfer RNA during protein translation. Some DNA or RNA-binding enzymes can recognize specific base pairing patterns that identify particular regulatory regions of genes. The ...
... biological systems. Pairing is also the mechanism by which codons on messenger RNA molecules are recognized by anticodons on transfer RNA during protein translation. Some DNA or RNA-binding enzymes can recognize specific base pairing patterns that identify particular regulatory regions of genes. The ...
Equilibrium STUDY GUIDE by Keshara Senanayake ---
... is Keq = [NH3][HCl] *please note though to reach equilibrium these substances (solids and liquids) are still important since their concentration don’t change their not included in the equilibrium constant The reaction quotient (Q) is used to determine if a reaction is at equilibrium. The reaction qu ...
... is Keq = [NH3][HCl] *please note though to reach equilibrium these substances (solids and liquids) are still important since their concentration don’t change their not included in the equilibrium constant The reaction quotient (Q) is used to determine if a reaction is at equilibrium. The reaction qu ...
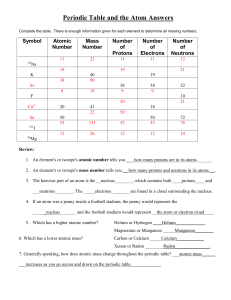
Periodic Table and the Atom Answers
... do this reaction with 125 grams of acetic acid and 275 grams of aluminum hydroxide. Two calculations are required. One determines the quantity of aluminum acetate that can be made with 125 grams of acetic acid and the other determines the quantity of aluminum acetate that can be made using 275 grams ...
... do this reaction with 125 grams of acetic acid and 275 grams of aluminum hydroxide. Two calculations are required. One determines the quantity of aluminum acetate that can be made with 125 grams of acetic acid and the other determines the quantity of aluminum acetate that can be made using 275 grams ...