Chemistry 12 is an intensive course, covering a great deal of
... 2. calculate the concentration of the positive and negative ions given the concentration of a solute in an aqueous solution ...
... 2. calculate the concentration of the positive and negative ions given the concentration of a solute in an aqueous solution ...
Mathematical Models of Ionic Flow Through Open Protein Channels
... short range forces and excluded volume effects, which are important for ionic flow through confined regions such as a protein channel. The second component of the force, not present at all in standard treatments, is the self induced force on a single discrete ion due to surface charges induced by th ...
... short range forces and excluded volume effects, which are important for ionic flow through confined regions such as a protein channel. The second component of the force, not present at all in standard treatments, is the self induced force on a single discrete ion due to surface charges induced by th ...
Redox Reactions C12-1-10
... Several types of chemical reactions were carried out in this laboratory session, some redox and some non-redox. Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to an ...
... Several types of chemical reactions were carried out in this laboratory session, some redox and some non-redox. Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to an ...
Learning Outcomes
... giant molecular substances, e.g. poly(ethene); sand (silicon dioxide); diamond; graphite in order to deduce their properties (c) compare the bonding and structures of diamond and graphite in order to deduce their properties such as electrical conductivity, lubricating or cutting action (candidates w ...
... giant molecular substances, e.g. poly(ethene); sand (silicon dioxide); diamond; graphite in order to deduce their properties (c) compare the bonding and structures of diamond and graphite in order to deduce their properties such as electrical conductivity, lubricating or cutting action (candidates w ...
Test-tube Reactions - University of Manitoba
... Several types of chemical reactions were carried out in this laboratory session, some redox and some non-redox. Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to an ...
... Several types of chemical reactions were carried out in this laboratory session, some redox and some non-redox. Remember that although redox reactions are common, not all chemical reactions are redox reactions. All redox reactions involve complete or partial transfer of electrons from one atom to an ...
here
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
Chemistry - Chillicothe City Schools
... Essential Question #3: How do I draw orbital diagrams for the elements in the first three periods? Essential Question #4: How do I write the electron configuration of an atom from its position on the periodic table? o Unit II Title: Intramolecular and Intermolecular Chemical Bonding Big Idea # ...
... Essential Question #3: How do I draw orbital diagrams for the elements in the first three periods? Essential Question #4: How do I write the electron configuration of an atom from its position on the periodic table? o Unit II Title: Intramolecular and Intermolecular Chemical Bonding Big Idea # ...
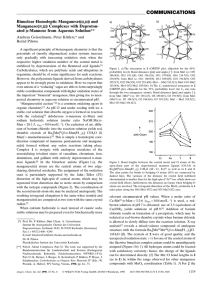
COMMUNICATIONS
... only gradually with increasing oxidation state when the respective higher oxidation number of the central metal is stabilized by deprotonation of the Brùnsted acid ligands.[1] Carbohydrates, which are polybasic acids and ubiquitous in organisms, should be of some significance for such reactions. How ...
... only gradually with increasing oxidation state when the respective higher oxidation number of the central metal is stabilized by deprotonation of the Brùnsted acid ligands.[1] Carbohydrates, which are polybasic acids and ubiquitous in organisms, should be of some significance for such reactions. How ...
CHAPTER 4: CHEMICAL QUANTITIES and AQUEOUS REACTIONS
... Solute (solid, liquid or gas) + Water (solvent) → Aqueous solution. If the aqueous solution conducts electric current, the solute is called as electrolytes. Electrolytes are classified into 3 types. ...
... Solute (solid, liquid or gas) + Water (solvent) → Aqueous solution. If the aqueous solution conducts electric current, the solute is called as electrolytes. Electrolytes are classified into 3 types. ...
Questa è la versione dell`autore dell`opera: [Chemical Reviews
... 2. Paramagnetic species in solids and at surfaces. In the field of surface science and heterogeneous catalysis, EPR techniques may be used to investigate i) either directly a radical, or ii) indirectly a diamagnetic system via a radical, often called spin probe. The latter is a molecule which, inter ...
... 2. Paramagnetic species in solids and at surfaces. In the field of surface science and heterogeneous catalysis, EPR techniques may be used to investigate i) either directly a radical, or ii) indirectly a diamagnetic system via a radical, often called spin probe. The latter is a molecule which, inter ...
NATIONAL HIGH SCHOOL CHEMISTRY EXAMINATION (1995
... b) the covalent bonds in bromine are stronger c) the intermolecular forces in bromine are stronger d) the covalent bonds in bromine are weaker e) the covalent bonds in bromine are more polar ...
... b) the covalent bonds in bromine are stronger c) the intermolecular forces in bromine are stronger d) the covalent bonds in bromine are weaker e) the covalent bonds in bromine are more polar ...
Chapter 4: Reactions in Aqueous Solution
... 1) Water is a very common solvent due to its wide availability and low cost (most of our world is water). 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with wat ...
... 1) Water is a very common solvent due to its wide availability and low cost (most of our world is water). 2) Many reactions take place in aqueous solution. The term aqueous means dissolved in water. 3) Hydration of solids in Water A) Solid dissolves (falls apart) through interaction of ions with wat ...
Document
... ion (0.099 nm). In fact, K+ and Ca2+ ions are isoelectronic and have the same number of electron shells. However, because Ca2+ ion has one more proton than K+ ion, the electrons of Ca2+ ion will experience a greater attractive force from the nucleus. This leads to a smaller ionic radius of Ca2+ ion. ...
... ion (0.099 nm). In fact, K+ and Ca2+ ions are isoelectronic and have the same number of electron shells. However, because Ca2+ ion has one more proton than K+ ion, the electrons of Ca2+ ion will experience a greater attractive force from the nucleus. This leads to a smaller ionic radius of Ca2+ ion. ...
Mobile ionic impurities in organic semiconductors
... metal/polymer contact resistances and by the ‘‘bulk’’ resistance of the film, the latter increasing with increasing L. The distinct difference between stress effects observed in short devices (Lⱗ5 m兲 and long devices (Lⲏ5 m兲 shown in Fig. 2 allows us to discriminate between contact and bulk effect ...
... metal/polymer contact resistances and by the ‘‘bulk’’ resistance of the film, the latter increasing with increasing L. The distinct difference between stress effects observed in short devices (Lⱗ5 m兲 and long devices (Lⲏ5 m兲 shown in Fig. 2 allows us to discriminate between contact and bulk effect ...
Sodium acetate method for determining CEC of cadmium
... distribution, CEC, amount of organic matter, total Cd concentration, and Cd content in various forms. The total amount of heavy metal was determined using the aqua regia digestion method. A sample of 3 g of dried soil was digested by slowly adding 21 mL concentrated hydrochloric acid and 7 mL nitric ...
... distribution, CEC, amount of organic matter, total Cd concentration, and Cd content in various forms. The total amount of heavy metal was determined using the aqua regia digestion method. A sample of 3 g of dried soil was digested by slowly adding 21 mL concentrated hydrochloric acid and 7 mL nitric ...
EOCT Physical Science Study Guide August 2008
... Students who actively study will learn and retain information longer. Active studying also helps you stay more alert and be more productive while learning new information. What is active studying? It can be anything that gets you to interact with the material you are studying. Here are a few suggest ...
... Students who actively study will learn and retain information longer. Active studying also helps you stay more alert and be more productive while learning new information. What is active studying? It can be anything that gets you to interact with the material you are studying. Here are a few suggest ...
Stoichiometry of Chemical Reactions
... The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water (aqueous solutions, as introduced in ...
... The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water (aqueous solutions, as introduced in ...
electrochemistry
... exothermic reaction is normally lost as heat. However, it can be trapped and converted into electrical energy if the reactants involved are not in direct contact with each other. In galvanic cells redox reactions is split into two halfreactions, each occurring in two separate compartments, called ha ...
... exothermic reaction is normally lost as heat. However, it can be trapped and converted into electrical energy if the reactants involved are not in direct contact with each other. In galvanic cells redox reactions is split into two halfreactions, each occurring in two separate compartments, called ha ...
Stoichiometry of Chemical Reactions
... The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water (aqueous solutions, as introduced in ...
... The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include s for solids, l for liquids, g for gases, and aq for substances dissolved in water (aqueous solutions, as introduced in ...
5. Formulae, equations and amounts of substance
... DEFINITION: The mole is the amount of substance in grams that has the same number of particles as there are atoms in 12 grams of carbon-12. DEFINITION: Relative atomic mass is the average mass of one atom compared to one twelfth of the mass of one atom of carbon-12 ...
... DEFINITION: The mole is the amount of substance in grams that has the same number of particles as there are atoms in 12 grams of carbon-12. DEFINITION: Relative atomic mass is the average mass of one atom compared to one twelfth of the mass of one atom of carbon-12 ...
AP Matter Class Packet Unit 5
... 4. Now you can see, that each acid on the left hand side produces a corresponding base on the right hand side. The base is called the conjugate base. Similarly, a base on the right hand side will produce a conjugate acid. These pairs are known as conjugate acid-base pairs. List the conjugate acid-ba ...
... 4. Now you can see, that each acid on the left hand side produces a corresponding base on the right hand side. The base is called the conjugate base. Similarly, a base on the right hand side will produce a conjugate acid. These pairs are known as conjugate acid-base pairs. List the conjugate acid-ba ...
Spatial resolution in atom probe tomography
... ‘resolution’ can be a source of confusion. Commonly, its use refers to the limit of resolution which is defined as the minimum distance between two objects that will permit their distinction as two separate points within the image produced by the microscope. However, the term is also used in relatio ...
... ‘resolution’ can be a source of confusion. Commonly, its use refers to the limit of resolution which is defined as the minimum distance between two objects that will permit their distinction as two separate points within the image produced by the microscope. However, the term is also used in relatio ...
Chem G 9
... neutrons will have different mass numbers and are called isotopes. Students should appreciate that a natural sample of an element is likely to contain a mixture of two or more isotopes. In determining the atomic mass of the element we must take into account that it is a mixture of isotopes with diff ...
... neutrons will have different mass numbers and are called isotopes. Students should appreciate that a natural sample of an element is likely to contain a mixture of two or more isotopes. In determining the atomic mass of the element we must take into account that it is a mixture of isotopes with diff ...
Topic 5: Counting electrons and holes
... incorporated into the crystal structure of the semiconductor. Either these impurities can be unintentional, due to lack of control during the growth of the semiconductor, or they can be added on purpose to provide free carriers in the semiconductor. The generation of free carriers requires not only ...
... incorporated into the crystal structure of the semiconductor. Either these impurities can be unintentional, due to lack of control during the growth of the semiconductor, or they can be added on purpose to provide free carriers in the semiconductor. The generation of free carriers requires not only ...