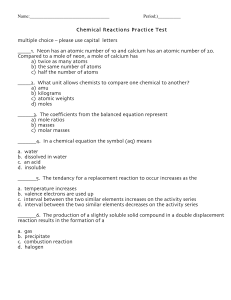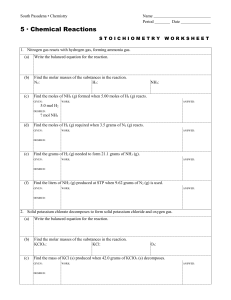
South Pasadena • Chemistry Name Period Date 5 · Chemical
... Find the volume of O2 (g) produced at STP when 42.0 grams of KClO3 (s) decomposes. GIVEN: ...
... Find the volume of O2 (g) produced at STP when 42.0 grams of KClO3 (s) decomposes. GIVEN: ...
Thermochem Practice Test
... a) that is exothermic, b) that has delta Suniv > 0, c) that has delta Ssys < 0, d) that has delta Ssurr > 0 2. Name the first law of thermodynamics. 3. Name the second law of thermodynamics. 4. Name the third law of thermodynamics 5. For a particular chemical reaction delta H = 5.5 kJ and delta S = ...
... a) that is exothermic, b) that has delta Suniv > 0, c) that has delta Ssys < 0, d) that has delta Ssurr > 0 2. Name the first law of thermodynamics. 3. Name the second law of thermodynamics. 4. Name the third law of thermodynamics 5. For a particular chemical reaction delta H = 5.5 kJ and delta S = ...
spontaneous processes
... equilibrium, we can go reversibly between reactants and products. 2. In any spontaneous process, the path between reactants and products is irreversible. 3. Thermodynamics refers to the direction of a reaction, not its speed. ...
... equilibrium, we can go reversibly between reactants and products. 2. In any spontaneous process, the path between reactants and products is irreversible. 3. Thermodynamics refers to the direction of a reaction, not its speed. ...
Chem BIG REVIEW - Jones-wiki
... A. The number of protons equals the number of electrons. B. The number of protons equals the number of neutrons. C. The number of neutrons equals the number of electrons. D. The number of neutrons is greater than the number of protons. 5. Consider the spectrum for the hydrogen atom. In which situati ...
... A. The number of protons equals the number of electrons. B. The number of protons equals the number of neutrons. C. The number of neutrons equals the number of electrons. D. The number of neutrons is greater than the number of protons. 5. Consider the spectrum for the hydrogen atom. In which situati ...
thermdyn - chemmybear.com
... lojoules per mole, yet the standard heat of formation of (d) Gf = Hf - TSf = 533.8 - (298)(-0.5239) kJ sodium chloride from its elements in their standard state is -411 kilojoules per mole. = -377.7 kJ (a) Name the factors that determine the magnitude of the standard heat of formation of solid ...
... lojoules per mole, yet the standard heat of formation of (d) Gf = Hf - TSf = 533.8 - (298)(-0.5239) kJ sodium chloride from its elements in their standard state is -411 kilojoules per mole. = -377.7 kJ (a) Name the factors that determine the magnitude of the standard heat of formation of solid ...
Chapter 9 Stoichiometry
... produced based on the limiting reactant. If everything in the reaction went according to plan, and all of the reactant(s) reacted, this is how much product should be made. This is NOT the same as the actual yield- amount that is produced based on an experiment Error occurs, so actual yield is ...
... produced based on the limiting reactant. If everything in the reaction went according to plan, and all of the reactant(s) reacted, this is how much product should be made. This is NOT the same as the actual yield- amount that is produced based on an experiment Error occurs, so actual yield is ...
Topic 1: Quantitative chemistry (12
... Be able to identify the ultraviolet, visible and infrared regions, and to describe the variation in wavelength, frequency and energy across the spectrum. TOK: Infrared and ultraviolet spectroscopy are dependent on technology for their existence. What are the knowledge implications of this? Distingui ...
... Be able to identify the ultraviolet, visible and infrared regions, and to describe the variation in wavelength, frequency and energy across the spectrum. TOK: Infrared and ultraviolet spectroscopy are dependent on technology for their existence. What are the knowledge implications of this? Distingui ...
Introduction to Computational Chemistry
... all three-center and four-center two-electron integrals. Replace by parameters to mimick experimental results (geometries and heats of formation). • solve secular equations just like in self-consistent field (HF) theory • typical methods: MNDO, MINDO, AM1, PM3 (order of development) ...
... all three-center and four-center two-electron integrals. Replace by parameters to mimick experimental results (geometries and heats of formation). • solve secular equations just like in self-consistent field (HF) theory • typical methods: MNDO, MINDO, AM1, PM3 (order of development) ...
Study of the self-diffusion coefficient in the water
... Self-diffusion coefficient in the water-methanol binary mixture was measured by NMR diffusion-order spectroscopy (DOSY) experiment [1] at different concentrations. The selfdiffusion coefficient of both water and methanol decreases exponentially as methanol mole fraction increases. This behavior is s ...
... Self-diffusion coefficient in the water-methanol binary mixture was measured by NMR diffusion-order spectroscopy (DOSY) experiment [1] at different concentrations. The selfdiffusion coefficient of both water and methanol decreases exponentially as methanol mole fraction increases. This behavior is s ...
Ionic Equations
... • Solutions of non-electrolytes have very low conductivities, since they produce no ions – Most molecular substances are non-electrolytes – Exceptions are acidic and basic molecules such as HCl(g) and NH3(g) ...
... • Solutions of non-electrolytes have very low conductivities, since they produce no ions – Most molecular substances are non-electrolytes – Exceptions are acidic and basic molecules such as HCl(g) and NH3(g) ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
File - Flipped Out Science with Mrs. Thomas!
... used to classify elements 2.2 Chemical Equations 5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 5F recognize whether a chemical equation containing coefficients is balanced or not and ho ...
... used to classify elements 2.2 Chemical Equations 5D recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing substances 5F recognize whether a chemical equation containing coefficients is balanced or not and ho ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.