Energy
... Electrical energy is actually derived from other sources of energy. Usually from the production of heat energy, through burning something, which is then used to boil water and produce steam which is used to turn a generator, which produces electrical energy. ...
... Electrical energy is actually derived from other sources of energy. Usually from the production of heat energy, through burning something, which is then used to boil water and produce steam which is used to turn a generator, which produces electrical energy. ...
Battle2005-Modeling of electromechanical systems.+
... adjacency matrix A by if branch α is incident on node i if branch α is anti-incident on node i otherwise ...
... adjacency matrix A by if branch α is incident on node i if branch α is anti-incident on node i otherwise ...
Physics Chapter 11
... 16. Bill throws a 10.0 g ball straight down from a height of 2.00 meters. The ball strikes the floor at a speed of 7.50 m/s. What was the initial speed of the ball? ...
... 16. Bill throws a 10.0 g ball straight down from a height of 2.00 meters. The ball strikes the floor at a speed of 7.50 m/s. What was the initial speed of the ball? ...
Final Exam Review
... Liquid: definite volume, not a definite shape - Liquids will take the shape of their container ...
... Liquid: definite volume, not a definite shape - Liquids will take the shape of their container ...
Energy - Effingham County Schools
... • Kinetic energy depends on two things: mass and speed. • Kinetic energy increases with speed. Consider a shopping cart with a certain speed. To make the cart move faster you need to apply a force to it. Applying a force means you have to do work. The higher the speed of the cart, the more energy it ...
... • Kinetic energy depends on two things: mass and speed. • Kinetic energy increases with speed. Consider a shopping cart with a certain speed. To make the cart move faster you need to apply a force to it. Applying a force means you have to do work. The higher the speed of the cart, the more energy it ...
force - SCIENCE
... The tendency of a body to resist a change in motion. An object at rest stays at rest. An object in motion stays in motion…unless acted on by an outside ...
... The tendency of a body to resist a change in motion. An object at rest stays at rest. An object in motion stays in motion…unless acted on by an outside ...
tide energy - WordPress.com
... Power is measured in joules per second (J/s) and one joule per second is known as a WATT (symbol W). WORKED EXAMPLE A man of mass 80 kg runs up 20 steps in 8 seconds. If each step is 25 cm high, what is his power? Weight of man = 80 kg x 10 = 800 N Total height in metres = 20 x 25 = 500 cm = 5.0 met ...
... Power is measured in joules per second (J/s) and one joule per second is known as a WATT (symbol W). WORKED EXAMPLE A man of mass 80 kg runs up 20 steps in 8 seconds. If each step is 25 cm high, what is his power? Weight of man = 80 kg x 10 = 800 N Total height in metres = 20 x 25 = 500 cm = 5.0 met ...
chapter7
... The quantity mgy is identified as the gravitational potential energy, Ug. Ug = mgy Units are joules (J) Is a scalar Work may change the gravitational potential energy of the system. Wext = DUg Potential energy is always associated with a system of two or more interacting objects. ...
... The quantity mgy is identified as the gravitational potential energy, Ug. Ug = mgy Units are joules (J) Is a scalar Work may change the gravitational potential energy of the system. Wext = DUg Potential energy is always associated with a system of two or more interacting objects. ...
Work, energy and momentum
... Force is measured in newtons (N). Distance moved is measured in metres (m). Work done is measured in joules (j). done against frictional forces is mainly ...
... Force is measured in newtons (N). Distance moved is measured in metres (m). Work done is measured in joules (j). done against frictional forces is mainly ...
"heat of fusion". - IES Al
... Consider the specific heat of copper, 0.385 J/g 0C. What this means is that it takes 0.385 Joules of heat to raise 1 gram of copper 1 degree Celsius. Thus, if we take 1 gram of copper at 25 0C and add 1 Joule of heat to it, we will find that the temperature of the copper will have risen to 26 0C. We ...
... Consider the specific heat of copper, 0.385 J/g 0C. What this means is that it takes 0.385 Joules of heat to raise 1 gram of copper 1 degree Celsius. Thus, if we take 1 gram of copper at 25 0C and add 1 Joule of heat to it, we will find that the temperature of the copper will have risen to 26 0C. We ...
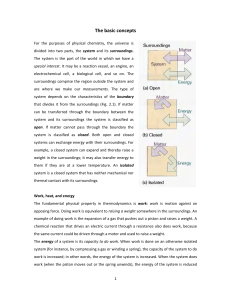
The basic concepts For the purposes of physical chemistry, the
... state of the system and is independent of how that state has been prepared. In other words, it is a function of the properties that determine the current state of the system. Changing anyone of the state variables, such as the pressure, results in a change in internal energy. The internal energy is ...
... state of the system and is independent of how that state has been prepared. In other words, it is a function of the properties that determine the current state of the system. Changing anyone of the state variables, such as the pressure, results in a change in internal energy. The internal energy is ...
Chapter 12 Notes
... a) a car with a mass of 1200 kg at the top of a 42 m high hill b) a 65 kg climber on top of Mount Everest (8800 m high) c) a .52 kg bird flying at an altitude of 550 m 2) Lake Mead, the reservoir about Hoover Dam, has a surface area of approximately 640 km². The top 1 m of water in the lake weighs a ...
... a) a car with a mass of 1200 kg at the top of a 42 m high hill b) a 65 kg climber on top of Mount Everest (8800 m high) c) a .52 kg bird flying at an altitude of 550 m 2) Lake Mead, the reservoir about Hoover Dam, has a surface area of approximately 640 km². The top 1 m of water in the lake weighs a ...
Work and Energy
... 5.3 Energy Transformations Energy transformations occur between different types of energy. — radiant energy — electrical energy — chemical energy — nuclear energy ...
... 5.3 Energy Transformations Energy transformations occur between different types of energy. — radiant energy — electrical energy — chemical energy — nuclear energy ...
AP1 Energy Review
... energy stored in a stretched or compressed spring. You just write out the equations, which are given. Easy as pie. (4) Calculate the potential energy of a single body in a uniform gravitational field. Use the U g mgh equation. b. You should understand conservation of energy so you can: (1) Identi ...
... energy stored in a stretched or compressed spring. You just write out the equations, which are given. Easy as pie. (4) Calculate the potential energy of a single body in a uniform gravitational field. Use the U g mgh equation. b. You should understand conservation of energy so you can: (1) Identi ...
Thermochemistry: Chemical Energy Chapter 8
... ΔHorxn = [(1 mol)(611 kJ/mol) + (4 mol)(410 kJ/mol) +(2 mol)(460 kJ/mol)]- [(1 mol)(350 kJ/mol) + (1 mol)( 350 kJ/mol) + (5 mol)(410 kJ/mol) + (1 mol)(460 kJ/mol)] ...
... ΔHorxn = [(1 mol)(611 kJ/mol) + (4 mol)(410 kJ/mol) +(2 mol)(460 kJ/mol)]- [(1 mol)(350 kJ/mol) + (1 mol)( 350 kJ/mol) + (5 mol)(410 kJ/mol) + (1 mol)(460 kJ/mol)] ...