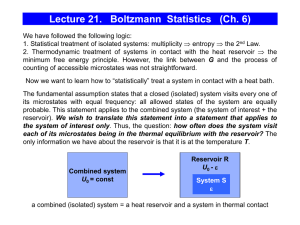
Lecture 21. Boltzmann Statistics (Ch. 6)
... minimum free energy principle. However, the link between G and the process of counting of accessible microstates was not straightforward. Now we want to learn how to “statistically” treat a system in contact with a heat bath. The fundamental assumption states that a closed (isolated) system visits e ...
... minimum free energy principle. However, the link between G and the process of counting of accessible microstates was not straightforward. Now we want to learn how to “statistically” treat a system in contact with a heat bath. The fundamental assumption states that a closed (isolated) system visits e ...
The Second Law - chem.uwec.edu
... heater attached to it, and water can be decomposed by the passage of an electric current. However, in each case we need to act in some way on the system to bring about the non-spontaneous change. There must be some feature of the world that accounts for the distinction between the two types of chang ...
... heater attached to it, and water can be decomposed by the passage of an electric current. However, in each case we need to act in some way on the system to bring about the non-spontaneous change. There must be some feature of the world that accounts for the distinction between the two types of chang ...
Map: Physics Curriculum Grade Level: 12 School Year: 2004-2005
... Energy may be stored in electric or magnetic fields. This energy may be transferred through conductors or space and may be converted to other forms of energy. ...
... Energy may be stored in electric or magnetic fields. This energy may be transferred through conductors or space and may be converted to other forms of energy. ...
an introduction to the concept of exergy and energy quality
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
the concept of exergy and energy quality
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
thermodynamics
... during chemical reactions when a fuel like methane, cooking gas or coal burns in air. The chemical energy may also be used to do mechanical work when a fuel burns in an engine or to provide electrical energy through a galvanic cell like dry cell. Thus, various forms of energy are interrelated and un ...
... during chemical reactions when a fuel like methane, cooking gas or coal burns in air. The chemical energy may also be used to do mechanical work when a fuel burns in an engine or to provide electrical energy through a galvanic cell like dry cell. Thus, various forms of energy are interrelated and un ...
Work and Energy LectureSlides
... thermal energy. And, because the sled’s height is constant, its gravitational potential energy is unchanged as well. Thus the work-energy equation is simply ΔK = W. We can therefore find the sled’s final kinetic energy, and hence its speed, by finding the work done by the racers as they push on the ...
... thermal energy. And, because the sled’s height is constant, its gravitational potential energy is unchanged as well. Thus the work-energy equation is simply ΔK = W. We can therefore find the sled’s final kinetic energy, and hence its speed, by finding the work done by the racers as they push on the ...
Potential Energy and Conservation of Energy
... and surface increase. The type of energy associated with temperature is internal energy, which we will study in detail in Chapter 20. Experience tells us that this internal energy cannot be transferred back to the kinetic energy of the book. In other words, the energy transformation is not reversibl ...
... and surface increase. The type of energy associated with temperature is internal energy, which we will study in detail in Chapter 20. Experience tells us that this internal energy cannot be transferred back to the kinetic energy of the book. In other words, the energy transformation is not reversibl ...