Ab initio Calculations of Optical Rotation
... within coupled cluster theory. The methods described within have been applied to several small chiral systems to assess the quality of the theoretically computed optical rotation. ...
... within coupled cluster theory. The methods described within have been applied to several small chiral systems to assess the quality of the theoretically computed optical rotation. ...
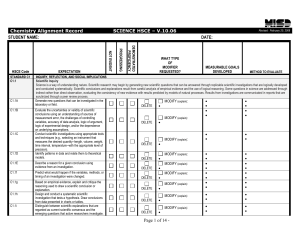
PC_Chemistry_Macomb_April08
... For each element, the arrangement of electrons surrounding the nucleus is unique. These electrons are found in different energy levels and can only move from a lower energy level (closer to nucleus) to a higher energy level (farther from nucleus) by absorbing energy in discrete packets. The energy c ...
... For each element, the arrangement of electrons surrounding the nucleus is unique. These electrons are found in different energy levels and can only move from a lower energy level (closer to nucleus) to a higher energy level (farther from nucleus) by absorbing energy in discrete packets. The energy c ...
Making and Breaking of Chemical Bonds
... is stabilized by transfer of the excess energy to a third (solvent) particle in a three–body collision. In contrast, the cage effect describes a situation where the presence of too many solvent particles can shield the reactants from each other thus leading to a reduced reactivity. • Finally, chapte ...
... is stabilized by transfer of the excess energy to a third (solvent) particle in a three–body collision. In contrast, the cage effect describes a situation where the presence of too many solvent particles can shield the reactants from each other thus leading to a reduced reactivity. • Finally, chapte ...
Quantum Mechanics for Pedestrians 1: Fundamentals
... Before attending the quantum mechanics course, the students have had among others an introduction to atomic physics: relevant phenomena, experiments and simple calculations should therefore be familiar to them. Nevertheless, experience has shown that at the start of the lectures, some students do no ...
... Before attending the quantum mechanics course, the students have had among others an introduction to atomic physics: relevant phenomena, experiments and simple calculations should therefore be familiar to them. Nevertheless, experience has shown that at the start of the lectures, some students do no ...
Spin Physics in Two-dimensional Systems Daniel Gosálbez Martínez
... It is difficult to enumerate all the properties that graphene has [19, 20, 33], here are highlight only some of the more relevant electronic properties[7]. One of them is that the electronic transport in graphene is remarkably more efficient than in any other semiconductor. The electron mobilities i ...
... It is difficult to enumerate all the properties that graphene has [19, 20, 33], here are highlight only some of the more relevant electronic properties[7]. One of them is that the electronic transport in graphene is remarkably more efficient than in any other semiconductor. The electron mobilities i ...
Bose-Einstein Condensation of Trapped Atomic Gases
... an enormous amount of theoretical and experimental work on the characterization of Bose-condensed gases. While the early work focussed on the equilibrium thermodynamics of condensates close to the phase transition, very soon the dynamical response of the condensate wavefunction to perturbations was ...
... an enormous amount of theoretical and experimental work on the characterization of Bose-condensed gases. While the early work focussed on the equilibrium thermodynamics of condensates close to the phase transition, very soon the dynamical response of the condensate wavefunction to perturbations was ...
Full-Text PDF
... These models have long been regarded as an important laboratory toolbox in numerous scientific fields from quantum chemistry to quantum information, mainly because they are completely integrable analogues of many-body systems due to their remarkable analytic properties. Moreover, Herschbach et al. [ ...
... These models have long been regarded as an important laboratory toolbox in numerous scientific fields from quantum chemistry to quantum information, mainly because they are completely integrable analogues of many-body systems due to their remarkable analytic properties. Moreover, Herschbach et al. [ ...
Algebraic Study on the Quantum Calogero Model
... China, August 19- 2-1 , 1996, ed. l\1.-L. Ge, Y. Saint-Au bin and L. Vinet (Springer- \"erlag). ...
... China, August 19- 2-1 , 1996, ed. l\1.-L. Ge, Y. Saint-Au bin and L. Vinet (Springer- \"erlag). ...
XVIIth International Workshop on Quantum Atomic and Molecular
... in molecular isomerization reactions and George Gamow (1928) on the theory of radioactive alpha decay, both being connected to ‘heavy’ particle motion (of the corresponding He nuclei or ‘atoms and molecules’) and thus fitting into the framework of the QAMTS workshops. Since then, numerous phenomena ...
... in molecular isomerization reactions and George Gamow (1928) on the theory of radioactive alpha decay, both being connected to ‘heavy’ particle motion (of the corresponding He nuclei or ‘atoms and molecules’) and thus fitting into the framework of the QAMTS workshops. Since then, numerous phenomena ...
Regularity and Approximability of Electronic Wave Functions
... Coulomb interaction forces in a field of clamped nuclei. Solutions of this equation depend on 3N variables, three spatial dimensions for each electron. Approximating the solutions is thus inordinately challenging, and it is conventionally believed that a reduction to simplified models, such as those ...
... Coulomb interaction forces in a field of clamped nuclei. Solutions of this equation depend on 3N variables, three spatial dimensions for each electron. Approximating the solutions is thus inordinately challenging, and it is conventionally believed that a reduction to simplified models, such as those ...
Quantum Chaos, Transport, and Decoherence in Atom
... to build up the cesium experiment from scratch, and he worked on the early kickedrotor experiments in Chapter 4. Bruce is not only laid back and very easy to work with, but also good at simply making things work. His insight, creativity, and curiosity made him a great asset to the lab as well as a g ...
... to build up the cesium experiment from scratch, and he worked on the early kickedrotor experiments in Chapter 4. Bruce is not only laid back and very easy to work with, but also good at simply making things work. His insight, creativity, and curiosity made him a great asset to the lab as well as a g ...