Task 1
... 4. The paradigm for a classic harmonic oscillator is a mass m attached to a spring of force constant k. The motion is governed by Hooke’s law. _________________________________________________________________________________________________________________ 5. The first term represents the kinetic en ...
... 4. The paradigm for a classic harmonic oscillator is a mass m attached to a spring of force constant k. The motion is governed by Hooke’s law. _________________________________________________________________________________________________________________ 5. The first term represents the kinetic en ...
MIT Physics Graduate General Exams
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
BasicQuantumMechanics20And22January
... Introduction to quantum mechanics (Chap.2) Quantum theory for semiconductors (Chap. 3) Allowed and forbidden energy bands (Chap. 3.1) Also refer to Appendices: Table B 2 (Conversion Factors), Table B.3 (Physical Constants), and Tables B.4 and B.5 Si, Ge, and GaAs key attributes and properties. We ...
... Introduction to quantum mechanics (Chap.2) Quantum theory for semiconductors (Chap. 3) Allowed and forbidden energy bands (Chap. 3.1) Also refer to Appendices: Table B 2 (Conversion Factors), Table B.3 (Physical Constants), and Tables B.4 and B.5 Si, Ge, and GaAs key attributes and properties. We ...
The Harmonic Oscilla..
... when operated on by the operator Ĥ , yield a constant (E) times the function itself. The wavefunctions should also be finite, single-valued, and continuous throughout the range from x → -∞ to x → ∞. As in the case of the free particle, Eq. (A) can be solved by expanding ψ in a power series, substit ...
... when operated on by the operator Ĥ , yield a constant (E) times the function itself. The wavefunctions should also be finite, single-valued, and continuous throughout the range from x → -∞ to x → ∞. As in the case of the free particle, Eq. (A) can be solved by expanding ψ in a power series, substit ...
Quantum Coherence between States with Even and Odd Numbers of Electrons
... 1. Let us consider a simple example where the interaction of the two parts of a total closed system can be described in terms of spinor external fields. Let there be two quantum dots and one electron which can occur with close energies in either of the dots and has a certain spin projection, specifi ...
... 1. Let us consider a simple example where the interaction of the two parts of a total closed system can be described in terms of spinor external fields. Let there be two quantum dots and one electron which can occur with close energies in either of the dots and has a certain spin projection, specifi ...
Some essential questions to be able to answer in Lecturer: McGreevy
... What information does this encode? Under what circumstances does the resulting ρA describe a pure state? 5. The density matrix encodes a probability distribution on state vectors: In its spectral representation X ρ= pa |aiha| a ...
... What information does this encode? Under what circumstances does the resulting ρA describe a pure state? 5. The density matrix encodes a probability distribution on state vectors: In its spectral representation X ρ= pa |aiha| a ...
BasicQuantumMechanics18And20January2017
... Introduction to quantum mechanics (Chap.2) Quantum theory for semiconductors (Chap. 3) Allowed and forbidden energy bands (Chap. 3.1) Also refer to Appendices: Table B 2 (Conversion Factors), Table B.3 (Physical Constants), and Tables B.4 and B.5 Si, Ge, and GaAs key attributes and properties. We ...
... Introduction to quantum mechanics (Chap.2) Quantum theory for semiconductors (Chap. 3) Allowed and forbidden energy bands (Chap. 3.1) Also refer to Appendices: Table B 2 (Conversion Factors), Table B.3 (Physical Constants), and Tables B.4 and B.5 Si, Ge, and GaAs key attributes and properties. We ...
Term paper
... Equation (4) is the strangest one. Actually, this comes from the Hartee-Fock method, which assumes that the wavefunction of a manyparticle(fermoinic) system is the product of the properly chosen wave functions of the individual particles. i.e ψ(x1 , x2 ) = χ(x1 )χ(x2 ). This is an ansatz and cannot ...
... Equation (4) is the strangest one. Actually, this comes from the Hartee-Fock method, which assumes that the wavefunction of a manyparticle(fermoinic) system is the product of the properly chosen wave functions of the individual particles. i.e ψ(x1 , x2 ) = χ(x1 )χ(x2 ). This is an ansatz and cannot ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
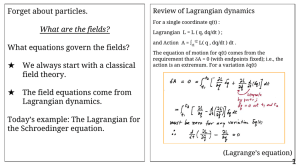
Forget about particles. What equations govern the fields? What are the fields?
... The equation of motion for q(t) comes from the requirement that δA = 0 (with endpoints fixed); i.e., the action is an extremum. For a variation δq(t) ...
... The equation of motion for q(t) comes from the requirement that δA = 0 (with endpoints fixed); i.e., the action is an extremum. For a variation δq(t) ...
Schrödinger`s Wave Mechanical Model
... wavelength which is a wave property, so he proved that matter could behave like waves. However, the wave properties of matter only become significant as the form of matter becomes smaller. This work resulted in what is known as the Wave-Particle Duality of Nature which states that matter and energy ...
... wavelength which is a wave property, so he proved that matter could behave like waves. However, the wave properties of matter only become significant as the form of matter becomes smaller. This work resulted in what is known as the Wave-Particle Duality of Nature which states that matter and energy ...
PH4038 - Lagrangian and Hamiltonian Dynamics
... the summary of deadlines etc on the School’s Students and Staff web pages. Five tutorial sheets will be issued over the semester in two week intervals. They contain questions that will deepen the understanding of the current topics in the lectures, and they are required to be handed in for marking. ...
... the summary of deadlines etc on the School’s Students and Staff web pages. Five tutorial sheets will be issued over the semester in two week intervals. They contain questions that will deepen the understanding of the current topics in the lectures, and they are required to be handed in for marking. ...
Chemistry 330 Chapter 11
... which waves interfere constructively and destructively in different directions. ...
... which waves interfere constructively and destructively in different directions. ...
... Eq. (2) one finds that A commutes with H and, therefore, if A does not depend on the time, then A is conserved. It should be pointed out that Refs. 8 and 9 also consider the Galilean transformations, which are related to a constant of motion that depends explicitly on the time (see Sec. 3.1, below). ...
Half-Life
... Initial Mass (1\2)x where x = number of half-lives Transmutation Reactions – Conversion of an atom of one element to an atom of another element. Transuranium Elements- All elements above atomic number 92 all undergo transmutation and do not occur naturally. Nuclear Fission versus Fusion Fission – Wh ...
... Initial Mass (1\2)x where x = number of half-lives Transmutation Reactions – Conversion of an atom of one element to an atom of another element. Transuranium Elements- All elements above atomic number 92 all undergo transmutation and do not occur naturally. Nuclear Fission versus Fusion Fission – Wh ...
Solution - UMD Physics
... a. What are the possible values obtained in a measurement of A? (2) b. Does a state exist in which both the results of a measurement of energy E and observable A can be predicted with certainty? Why or why not? (2) c. Two measurements of A are carried out, separated in time by t. If the result of th ...
... a. What are the possible values obtained in a measurement of A? (2) b. Does a state exist in which both the results of a measurement of energy E and observable A can be predicted with certainty? Why or why not? (2) c. Two measurements of A are carried out, separated in time by t. If the result of th ...