Particle in the box
... the edges of the box yields spatial boundary conditions. We seek then solutions of the time-dependent Schrödinger equation: ...
... the edges of the box yields spatial boundary conditions. We seek then solutions of the time-dependent Schrödinger equation: ...
Document
... 21. The __________ _____________ tells you how many electrons an atom must gain, lose, or share to become stable. 22. Numbers that precede symbols and formulas in a chemical equation are ______________. 23. A chemical reaction in which two or more substances combine to form another substance is call ...
... 21. The __________ _____________ tells you how many electrons an atom must gain, lose, or share to become stable. 22. Numbers that precede symbols and formulas in a chemical equation are ______________. 23. A chemical reaction in which two or more substances combine to form another substance is call ...
Journal of Babylon University/Pure and Applied Sciences/ No.(6
... fermion pairs. The other idea is that the fermion pairs couple to form bosons, carrying angular momentum (J). The energies (εs and εd), and the interactions of the s and d bosons, predict the low-lying excitations in the nucleus. There is 1 available magnetic substate for the s boson, determined by ...
... fermion pairs. The other idea is that the fermion pairs couple to form bosons, carrying angular momentum (J). The energies (εs and εd), and the interactions of the s and d bosons, predict the low-lying excitations in the nucleus. There is 1 available magnetic substate for the s boson, determined by ...
VI Zagrebaev and VV Samarin
... the set of coupled Schrödinger equations that simulates the coupling of channels in the nearbarrier fusion of heavy nuclei have been proposed in recent years. These algorithms rely either on employing some approximate method to diagonalize the coupling matrix at the barrier [1] or on directly constr ...
... the set of coupled Schrödinger equations that simulates the coupling of channels in the nearbarrier fusion of heavy nuclei have been proposed in recent years. These algorithms rely either on employing some approximate method to diagonalize the coupling matrix at the barrier [1] or on directly constr ...
PS#4
... 3. Use the Slater determinant to arrive at a wave function to describe the ground state of a two-electron system such as He. Express the resulting wave function in terms of the 1s spatial wave function for each electron [ 1s 1 and 1s 2 ], and of the spin wave functions for each electron 1, ...
... 3. Use the Slater determinant to arrive at a wave function to describe the ground state of a two-electron system such as He. Express the resulting wave function in terms of the 1s spatial wave function for each electron [ 1s 1 and 1s 2 ], and of the spin wave functions for each electron 1, ...
atomsagain
... n,l ,m r, , Rn,l r Yl .m , The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the stat ...
... n,l ,m r, , Rn,l r Yl .m , The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the stat ...
Schrödinger Equation
... the wavefunction which predicts analytically and precisely the probability of events or outcome. The detailed outcome is not strictly determined, but given a large number of events, the Schrödinger equation will predict the distribution of results. ...
... the wavefunction which predicts analytically and precisely the probability of events or outcome. The detailed outcome is not strictly determined, but given a large number of events, the Schrödinger equation will predict the distribution of results. ...
Test #5 Review
... Changing from gas to liquid phase is called . . . condensation. Does a liquid absorb or give off energy when freezing? Energy is given off. ...
... Changing from gas to liquid phase is called . . . condensation. Does a liquid absorb or give off energy when freezing? Energy is given off. ...
Document
... Though there are discrete levels of electronic excited states in a molecule, we do not observe absorption lines but broad peaks; th e change in vibrational and rotational energy levels during absorption of light leads to peaks containing vibrational and rotational fine structure. Due to additional ...
... Though there are discrete levels of electronic excited states in a molecule, we do not observe absorption lines but broad peaks; th e change in vibrational and rotational energy levels during absorption of light leads to peaks containing vibrational and rotational fine structure. Due to additional ...
Chapter 7 Section 1 - School District 27J
... Because we cannot pinpoint exactly where an electron is on the “surface” of an atom, we refer to its position as an electron cloud. The chemical behavior and properties of any 2-subtances are determined by the number of these electrons around the nucleus ...
... Because we cannot pinpoint exactly where an electron is on the “surface” of an atom, we refer to its position as an electron cloud. The chemical behavior and properties of any 2-subtances are determined by the number of these electrons around the nucleus ...
The study of biology can help you better understand
... Write noble gas notation for electrons configuration for the following atoms: d orbital can hold maximum 10 electrons. K ________________________________________________________________ Ca ________________________________________________________________ ...
... Write noble gas notation for electrons configuration for the following atoms: d orbital can hold maximum 10 electrons. K ________________________________________________________________ Ca ________________________________________________________________ ...
kinetic energy of photoelectrons (eV)
... 5) The photoelectrons are emitted immediately (classical physics predicted time delays of weeks for very faint light) 6) The brightness of the light increased the amount of photoelectrons, but did not change the kinetic energy ...
... 5) The photoelectrons are emitted immediately (classical physics predicted time delays of weeks for very faint light) 6) The brightness of the light increased the amount of photoelectrons, but did not change the kinetic energy ...
Forms of Energy
... • Potential: stored energy and energy of position • Kinetic: motion of waves, electrons, atoms, molecules and substances. ...
... • Potential: stored energy and energy of position • Kinetic: motion of waves, electrons, atoms, molecules and substances. ...
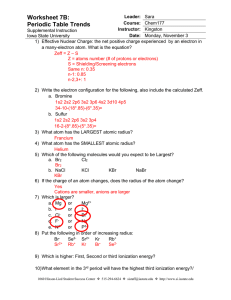
worksheet 7b answers - Iowa State University
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
... Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = Z – S Z = atoms number (# of protons or electrons) S = Shielding/Screening electrons Same n: 0.35 n-1: 0.85 n-2,3+: 1 ...
Chemical Physics High-spin-low-spin transitions in Fe(II) complexes
... state spin depends on the balance between the electron repulsion of d-electrons and their interaction with the ligands which provide some external field. The excitations of the ligands have much larger energies than those in the d-shell and incidentally they have a closed electronic shell so that th ...
... state spin depends on the balance between the electron repulsion of d-electrons and their interaction with the ligands which provide some external field. The excitations of the ligands have much larger energies than those in the d-shell and incidentally they have a closed electronic shell so that th ...