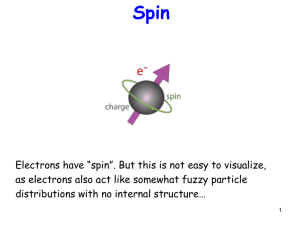
CHAPTER 4: Structure of the Atom
... In the present first part of the paper the mechanism of the binding of electrons by a positive nucleus is discussed in relation to Planck’s theory. It will be shown that it is possible from the point of view taken to account in a simple way for the law of the line spectrum of hydrogen. - Niels Bohr, ...
... In the present first part of the paper the mechanism of the binding of electrons by a positive nucleus is discussed in relation to Planck’s theory. It will be shown that it is possible from the point of view taken to account in a simple way for the law of the line spectrum of hydrogen. - Niels Bohr, ...
Pdf
... lnG in a cluster series; relations (11), (12), and (13) in turn allow evaluation of all thermodynamic properties. ...
... lnG in a cluster series; relations (11), (12), and (13) in turn allow evaluation of all thermodynamic properties. ...
Atomic Structure and Periodicity
... The positive nuclei are surrounded by a “sea” of mobile electrons Their valence shells have the same energy The closer an electron is to the nucleus, the more difficult it is to remove A sodium atom does not have as many valence electrons as a chlorine atom does. 3s electrons are lower in energy tha ...
... The positive nuclei are surrounded by a “sea” of mobile electrons Their valence shells have the same energy The closer an electron is to the nucleus, the more difficult it is to remove A sodium atom does not have as many valence electrons as a chlorine atom does. 3s electrons are lower in energy tha ...
Physical Science
... Drill bits are often coated to make them harder, so that they can drill through many different types of materials. A coating of which element from the table would make a drill bit the hardest? A. Iron B. Boron C. Calcium D. Magnesium A 500 ...
... Drill bits are often coated to make them harder, so that they can drill through many different types of materials. A coating of which element from the table would make a drill bit the hardest? A. Iron B. Boron C. Calcium D. Magnesium A 500 ...
hydrogen
... Ions such as He+ and Li2+ are hydrogen-like since they also have only a single electron. In each case the mass of the electron is much less the nuclear mass, therefore, we will assume a stationary nucleus exerting an attractive force that binds the electron. This is the Coulomb force with correspond ...
... Ions such as He+ and Li2+ are hydrogen-like since they also have only a single electron. In each case the mass of the electron is much less the nuclear mass, therefore, we will assume a stationary nucleus exerting an attractive force that binds the electron. This is the Coulomb force with correspond ...
Contents
... and the density functional theory. The last is the framework for the Thomas-Fermi approximation and its systematic density-gradient corrections. It is also the basis for the local-density approximation, which has been widely used, especially by solid state physicists and quantum chemists. By a sligh ...
... and the density functional theory. The last is the framework for the Thomas-Fermi approximation and its systematic density-gradient corrections. It is also the basis for the local-density approximation, which has been widely used, especially by solid state physicists and quantum chemists. By a sligh ...
Phase Transitions of Dirac Electrons Observed in Bismuth
... equivalent effects in vacuum, one would need intense magnetic fields found only on the surface of a neutron star). The vast difference in scale introduces new electronic phenomena that can be studied in the laboratory. A second important difference arises from interaction effects. Whereas the electr ...
... equivalent effects in vacuum, one would need intense magnetic fields found only on the surface of a neutron star). The vast difference in scale introduces new electronic phenomena that can be studied in the laboratory. A second important difference arises from interaction effects. Whereas the electr ...
Chemistry FINAL: CONTENT Review Packet
... h. a counting unit; 6.022 x 1023 i. positively charged subatomic particle j. subatomic particle with no charge ...
... h. a counting unit; 6.022 x 1023 i. positively charged subatomic particle j. subatomic particle with no charge ...
Chemistry Comes Alive: Part A
... • Chemical energy—stored in bonds of chemical substances • Electrical energy—results from movement of charged particles • Mechanical energy—directly involved in moving matter • Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
... • Chemical energy—stored in bonds of chemical substances • Electrical energy—results from movement of charged particles • Mechanical energy—directly involved in moving matter • Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
Chemistry FINAL: CONTENT Review Packet
... retains the properties of that element h. a counting unit; 6.022 x 1023 i. positively charged subatomic particle j. subatomic particle with no charge ...
... retains the properties of that element h. a counting unit; 6.022 x 1023 i. positively charged subatomic particle j. subatomic particle with no charge ...
CHAPTER 2. LAGRANGIAN QUANTUM FIELD THEORY §2.1
... consistent manner. This will be done in the framework of specific models. In fact some fields obey equations of motion which are not derivable from local Lagrangians, their field equations are said to have anomalous terms in them. The form of anomalies in QFT is an extremely important subject since ...
... consistent manner. This will be done in the framework of specific models. In fact some fields obey equations of motion which are not derivable from local Lagrangians, their field equations are said to have anomalous terms in them. The form of anomalies in QFT is an extremely important subject since ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Use the periodic table to predict number of valence electrons Use the periodic table to predict the electron configuration of an element Indicate the probable pattern of electrons around the nucleus of any atom using the electron configuration notation Distinguish between s and p orbitals Distinguis ...
... Use the periodic table to predict number of valence electrons Use the periodic table to predict the electron configuration of an element Indicate the probable pattern of electrons around the nucleus of any atom using the electron configuration notation Distinguish between s and p orbitals Distinguis ...
Chapter 3
... The Bohr was limited. Erwin Schodinger in 1926 developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. This is known as quantum mechanics. ...
... The Bohr was limited. Erwin Schodinger in 1926 developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated. This is known as quantum mechanics. ...