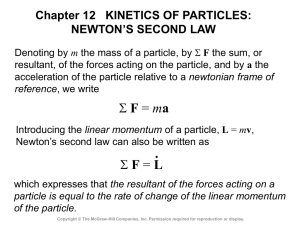
Atoms and Energies
... When the capacitor is fully charged and no more electrons can be added, the potential energy of the capacitor equals the maximum kinetic energy of the electrons trying to leave the original plate The potential difference on the capacitor at this point is called the stopping potential Vs for the elec ...
... When the capacitor is fully charged and no more electrons can be added, the potential energy of the capacitor equals the maximum kinetic energy of the electrons trying to leave the original plate The potential difference on the capacitor at this point is called the stopping potential Vs for the elec ...
Fermion Mixtures in an Optical Lattice
... Competition between kinetic and repulsive energy Bose-Einstein Condensate: kinetic energy wins ...
... Competition between kinetic and repulsive energy Bose-Einstein Condensate: kinetic energy wins ...
ATOMIC PHYSICS
... 6) Determine the momentum of an electron travelling at 2.43x107m/s. Using the momentum determine the theoretical wavelength of the electron. ...
... 6) Determine the momentum of an electron travelling at 2.43x107m/s. Using the momentum determine the theoretical wavelength of the electron. ...
Properties of photons with similarities to waves and or particles
... Quantum mechanics is the fundamental theory of small scale phenomena, such as the properties of electrons, photons and atomic interactions. Classical physics deals with continuous quantities, whereas quantum mechanics shows that on the very small scale, all quantities are discrete or quantised. In p ...
... Quantum mechanics is the fundamental theory of small scale phenomena, such as the properties of electrons, photons and atomic interactions. Classical physics deals with continuous quantities, whereas quantum mechanics shows that on the very small scale, all quantities are discrete or quantised. In p ...