Topic 11 — relativity - energy and momentum — Use the
... Topic 11 — relativity - energy and momentum — Use the fundamental relations between the mass, velocity, energy and momentum of a particle and conservation of energy and momentum to solve problems in relativistic kinematics and to simplify calculations involving space and time. Use 4-vectors and the ...
... Topic 11 — relativity - energy and momentum — Use the fundamental relations between the mass, velocity, energy and momentum of a particle and conservation of energy and momentum to solve problems in relativistic kinematics and to simplify calculations involving space and time. Use 4-vectors and the ...
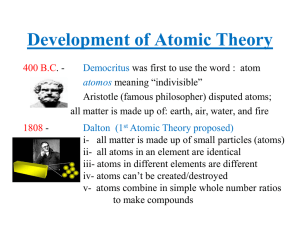
Development of Atomic Theory
... (stationary state) but could gain energy and jump into a higher orbit (excited or transition state) or could lose energy and fall to a lower orbit, or the lowest orbit (ground state) ...
... (stationary state) but could gain energy and jump into a higher orbit (excited or transition state) or could lose energy and fall to a lower orbit, or the lowest orbit (ground state) ...
Atomic Theory
... He believed that matter was made up of tiny particles called atoms. He also believed that matter could not be created, destroyed, or further divided. His theory was met with criticism from other influential philosophers such as Aristotle. His theory was eventually rejected because it was not support ...
... He believed that matter was made up of tiny particles called atoms. He also believed that matter could not be created, destroyed, or further divided. His theory was met with criticism from other influential philosophers such as Aristotle. His theory was eventually rejected because it was not support ...
Modern Physics
... e.g. Average A Level score is same for females as for males. Not an exact symmetry. ...
... e.g. Average A Level score is same for females as for males. Not an exact symmetry. ...
Transparancies for Revision Lecture - University of Manchester
... Interacts with magnetic field, U=mlBB Zeeman effect gives splitting of states ...
... Interacts with magnetic field, U=mlBB Zeeman effect gives splitting of states ...
What is the Higgs? - University of Manchester
... E.g. At Fermilab the collision of a single proton and antiproton is sufficiently energetic to produce over 2000 protons. At CERN, the electron and positron collided with sufficient energy to produce over 200 protons (electrons are more than 1000 times lighter than a proton!) ...
... E.g. At Fermilab the collision of a single proton and antiproton is sufficiently energetic to produce over 2000 protons. At CERN, the electron and positron collided with sufficient energy to produce over 200 protons (electrons are more than 1000 times lighter than a proton!) ...
Einstein`s Miraculous Year
... frequency needed for the photoelectric effect to work. Every metal has a minimum ...
... frequency needed for the photoelectric effect to work. Every metal has a minimum ...
Life in the Higgs condensate, where electrons have mass
... mass of an electron were zero there would be no atoms, and the formation of ordinary matter would be impossible without the Higgs condensate. (However, about 99% of the mass of an atom or human body results from the kinetic energy of quarks and gluons as they whiz around relativistically inside the ...
... mass of an electron were zero there would be no atoms, and the formation of ordinary matter would be impossible without the Higgs condensate. (However, about 99% of the mass of an atom or human body results from the kinetic energy of quarks and gluons as they whiz around relativistically inside the ...
Oct 6
... quarks and leptons, are currently believed to be the “atomic” (un-cut-able) elements of the universe ...
... quarks and leptons, are currently believed to be the “atomic” (un-cut-able) elements of the universe ...
6. Divisibility of atoms: from radioactivity to particle physics
... Further required particle: Higgs bosons (to explain mass difference of photons (zero mass) and W+,W-, Z0) ...
... Further required particle: Higgs bosons (to explain mass difference of photons (zero mass) and W+,W-, Z0) ...
T
... observables whose SM predictions are well known and that are expected to be extremely sensitive to a wide range of beyond the SM theories, e.g., supersymmetry. The magic of quantum mechanics permits particles that are too massive to be produced in the lab, even at the LHC, to make significant contri ...
... observables whose SM predictions are well known and that are expected to be extremely sensitive to a wide range of beyond the SM theories, e.g., supersymmetry. The magic of quantum mechanics permits particles that are too massive to be produced in the lab, even at the LHC, to make significant contri ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.