13. nuclear
... If you see an atom AND one of the high energy particles on left side of arrow combining to make a new element on the right side of arrow, then it is an artificial transmutation!! ...
... If you see an atom AND one of the high energy particles on left side of arrow combining to make a new element on the right side of arrow, then it is an artificial transmutation!! ...
AQA A Physics - Particle Physics
... introduction to group theory is beyond the scope of this document. In 1968 at the Stanford Linear Accelerator Centre, deep inelastic scattering experiments demonstrated that the proton contains much smaller ‘point-like’ objects so is not an elementary particle itself. It was some time before these o ...
... introduction to group theory is beyond the scope of this document. In 1968 at the Stanford Linear Accelerator Centre, deep inelastic scattering experiments demonstrated that the proton contains much smaller ‘point-like’ objects so is not an elementary particle itself. It was some time before these o ...
New analysis shows a way to self
... particles, such as electrons, in terms of a wave structure. (In quantum mechanics, waves and particles are considered to be two aspects of the same physical phenomena). By manipulating the wave structure, the team found, it should be possible to cause electrons to behave in unusual and counterintuit ...
... particles, such as electrons, in terms of a wave structure. (In quantum mechanics, waves and particles are considered to be two aspects of the same physical phenomena). By manipulating the wave structure, the team found, it should be possible to cause electrons to behave in unusual and counterintuit ...
An Introduction To Particle Accelerators
... Why do we need particle accelerators? If particles have large velocity, the wavelength decreases. So they can be used to study atomic spacing. High energy particles can be smashed into each other, allowing other particles to be ...
... Why do we need particle accelerators? If particles have large velocity, the wavelength decreases. So they can be used to study atomic spacing. High energy particles can be smashed into each other, allowing other particles to be ...
Detecting particles in particle physics
... Exercise The exercise you are going to do is to try to recognise different types of particles and neutrino interactions using a program The human brain is one of the best pattern matching machines ever evolved. However, even we will get some of these wrong. Imagine how much harder it is to ask a co ...
... Exercise The exercise you are going to do is to try to recognise different types of particles and neutrino interactions using a program The human brain is one of the best pattern matching machines ever evolved. However, even we will get some of these wrong. Imagine how much harder it is to ask a co ...
Answers to Coursebook questions – Chapter J1
... electrons occupying that state be differentiated in some way. The inner shell has no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the othe ...
... electrons occupying that state be differentiated in some way. The inner shell has no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the othe ...
Chapter 17 - Probing Deep into Matter
... Electrons in atoms have particular wavelengths, so have particular energies. Work out k.e. and p.e. (see page 94). SoftAct 150S 'Sizing up a hydrogen atom' Lesson 8: Energy levels and transitions Objectives ...
... Electrons in atoms have particular wavelengths, so have particular energies. Work out k.e. and p.e. (see page 94). SoftAct 150S 'Sizing up a hydrogen atom' Lesson 8: Energy levels and transitions Objectives ...
Precursors to Modern Physics
... atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with the same quantum numbers, what is the trend of the ...
... atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with the same quantum numbers, what is the trend of the ...
ATOMIC PHYSICS
... invalidates the classical model of the atom describe that each element has a unique line spectrum explain, qualitatively, the characteristics of, and the conditions necessary to produce, continuous line-emission and line-absorption spectra explain, qualitatively, the concept of stationary states and ...
... invalidates the classical model of the atom describe that each element has a unique line spectrum explain, qualitatively, the characteristics of, and the conditions necessary to produce, continuous line-emission and line-absorption spectra explain, qualitatively, the concept of stationary states and ...
Electron discovered 1897, Thomson Atom model 1913, Bohr
... 1897, Thomson Atom model 1913, Bohr, Rutherford ...
... 1897, Thomson Atom model 1913, Bohr, Rutherford ...
When Symmetry Breaks Down - School of Natural Sciences
... But in our modern understanding, which is based on something called the ‘standard model’ of particle physics, the two are perfectly in parallel. For example, electromagnetism is described by Maxwell’s equations, and the weak interactions are described by a quite similar, albeit nonlinear, set of equ ...
... But in our modern understanding, which is based on something called the ‘standard model’ of particle physics, the two are perfectly in parallel. For example, electromagnetism is described by Maxwell’s equations, and the weak interactions are described by a quite similar, albeit nonlinear, set of equ ...
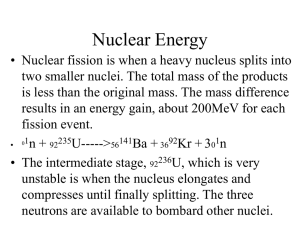
Nuclear Energy - Eastside Physics
... • The three quarks are colored red blue and green. The antquarks are anti-red anti-blue and antigreen. Baryons consist of the three different colored quarks and mesons consist of one color and an anti quark of the anti-color. Both baryons and meson are colorless. The strong force between quarks is c ...
... • The three quarks are colored red blue and green. The antquarks are anti-red anti-blue and antigreen. Baryons consist of the three different colored quarks and mesons consist of one color and an anti quark of the anti-color. Both baryons and meson are colorless. The strong force between quarks is c ...
Document
... absorbed by a nucleus, and then “exploded” into “stars” (D.H. Perkins was one who observed these!) • The positive particles seemed to stop and then decay into the previously-seen muons • These had a similar mass to the mesons, but clearly had different interactions m 135 MeV ...
... absorbed by a nucleus, and then “exploded” into “stars” (D.H. Perkins was one who observed these!) • The positive particles seemed to stop and then decay into the previously-seen muons • These had a similar mass to the mesons, but clearly had different interactions m 135 MeV ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.