Basics of Electron Storage Rings
... (rf fields) has become exceptionally effective for the acceleration of charged particles. When the particle passes the rf cavity, it gets energy from the rf field. - In the electron storage ring, the energy of the particle is lost by U0 per turn due to the emission of synchrotron light. The particle ...
... (rf fields) has become exceptionally effective for the acceleration of charged particles. When the particle passes the rf cavity, it gets energy from the rf field. - In the electron storage ring, the energy of the particle is lost by U0 per turn due to the emission of synchrotron light. The particle ...
1. The graph shows how the displacement varies
... An identical mass is attached to an identical spring. The maximum displacement is 2A. Assuming this spring obeys Hooke’s law, which of the following gives the correct time period and total energy? New time period ...
... An identical mass is attached to an identical spring. The maximum displacement is 2A. Assuming this spring obeys Hooke’s law, which of the following gives the correct time period and total energy? New time period ...
PHYS 1443 – Section 501 Lecture #1
... • Cannot adequately describe small scale phenomena with classical mechanics and E&M • The study of atomic structure led to quantum mechanics (QM) – Long range E&M force is responsible for holding atoms together – Yet it is sufficiently weak that QM can be used to reliably predict properties of atoms ...
... • Cannot adequately describe small scale phenomena with classical mechanics and E&M • The study of atomic structure led to quantum mechanics (QM) – Long range E&M force is responsible for holding atoms together – Yet it is sufficiently weak that QM can be used to reliably predict properties of atoms ...
View - Workshops+SJCOE Workshop Management
... positive. The question of the stability of the atom proposed need not be considered at this stage, for this will obviously depend upon the minute structure of the atom, and on the motion of the constituent charged parts. In order to form some idea of the forces required {o deflect an ~ particle thro ...
... positive. The question of the stability of the atom proposed need not be considered at this stage, for this will obviously depend upon the minute structure of the atom, and on the motion of the constituent charged parts. In order to form some idea of the forces required {o deflect an ~ particle thro ...
Interaction of Radiation with Matter
... emitted at a well-defined angle cosθ = 1/βn w.r.t the trajectory of the particle. The energy loss increases with β, however even at relativistic energies the energy loss is small compared to collision loss. Advantage : very accurate β measurement of relativistic particles, since cone angle of radiat ...
... emitted at a well-defined angle cosθ = 1/βn w.r.t the trajectory of the particle. The energy loss increases with β, however even at relativistic energies the energy loss is small compared to collision loss. Advantage : very accurate β measurement of relativistic particles, since cone angle of radiat ...
You Are What You Think
... limited by time or space. You can think of something that is not physically present; you can think of something that happened or will happen; and you can think of something that does not exist at all. As matter, thought exists as molecules that form, break down, reform, and communicate with each oth ...
... limited by time or space. You can think of something that is not physically present; you can think of something that happened or will happen; and you can think of something that does not exist at all. As matter, thought exists as molecules that form, break down, reform, and communicate with each oth ...
• Introduction R ⇒
... A conductor is a material which allows charged particles to move freely through it and these particles carry the charge on the conductor. For example, a metal may be considered to be a structure of positive ions occupying fixed positions on a lattice with the free electrons moving between them. The ...
... A conductor is a material which allows charged particles to move freely through it and these particles carry the charge on the conductor. For example, a metal may be considered to be a structure of positive ions occupying fixed positions on a lattice with the free electrons moving between them. The ...
PHY 184 lecture 1 - MSU Department of Physics and Astronomy
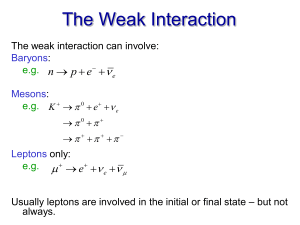
... In the 20th century, two more forces were discovered • The weak force and the strong force – inside the atomic nucleus ...
... In the 20th century, two more forces were discovered • The weak force and the strong force – inside the atomic nucleus ...
Ch4_S1A
... Dalton’s Atomic Theory Dalton’s Theory • All elements are composed of atoms. • All atoms of the same element have the same mass, and atoms of different elements have different masses. • Compounds contain atoms of more than one element. • In a particular compound, atoms of different elements always c ...
... Dalton’s Atomic Theory Dalton’s Theory • All elements are composed of atoms. • All atoms of the same element have the same mass, and atoms of different elements have different masses. • Compounds contain atoms of more than one element. • In a particular compound, atoms of different elements always c ...
BCIT Fall 2012 Chem 3615 Exam #2
... Section II: Short answer calculations do not need to be shown (16 points total). ...
... Section II: Short answer calculations do not need to be shown (16 points total). ...
Name
... Fission splits a large nucleus into smaller nuclei. Fusion combines two small nuclei into one larger one. 41. Briefly describe what happens that allows you to see colors in the flame tests and the gas tubes. When energy is added to an atom, an electron jumps to a higher energy level (excited state). ...
... Fission splits a large nucleus into smaller nuclei. Fusion combines two small nuclei into one larger one. 41. Briefly describe what happens that allows you to see colors in the flame tests and the gas tubes. When energy is added to an atom, an electron jumps to a higher energy level (excited state). ...
Physics and the Quantum Mechanical Model
... Therefore, they gain or lose energy in packages called quanta Includes the uncertainty principle ...
... Therefore, they gain or lose energy in packages called quanta Includes the uncertainty principle ...
Why do elements combine
... Why do Elements Combine? • Elements seek a stable outer energy level ⇒ Full or 8 electrons • Elements can borrow or share electrons ⇒ Metals tend to lend electrons ⇒ Non-metals tend to borrow • The number of electrons an element is likely to lend or borrow is known as its valence A Review... • Charg ...
... Why do Elements Combine? • Elements seek a stable outer energy level ⇒ Full or 8 electrons • Elements can borrow or share electrons ⇒ Metals tend to lend electrons ⇒ Non-metals tend to borrow • The number of electrons an element is likely to lend or borrow is known as its valence A Review... • Charg ...
O Strong-Arming Electron Spin Dynamics
... spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two new methods for single spin control that have been developed by my group at Princeton. The first method is based on quantum interference and implements spin-interferom ...
... spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two new methods for single spin control that have been developed by my group at Princeton. The first method is based on quantum interference and implements spin-interferom ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.