Heat Pipe Background
... experimental settings at key locations (e.g. axial-settings) Ensure testing conditions are uniform across experimental settings Use more than one unit per experimental setting ...
... experimental settings at key locations (e.g. axial-settings) Ensure testing conditions are uniform across experimental settings Use more than one unit per experimental setting ...
An Approach to a Zero
... Earth has higher thermal conductivity than many insulating materials, but it can make up for that with thickness. Let’s take the limit of finding a 200 m2 cave, so far underground that the cave temperature is the yearly average air temperature, which we can take to be 5 C. This will also be the temp ...
... Earth has higher thermal conductivity than many insulating materials, but it can make up for that with thickness. Let’s take the limit of finding a 200 m2 cave, so far underground that the cave temperature is the yearly average air temperature, which we can take to be 5 C. This will also be the temp ...
PPT Slide Show
... • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
... • Heat transfer explains the transfer of thermal energy, between physical systems depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation. ...
Chapter Two Atoms & The Periodic Table
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
... Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) ...
Chap #13
... Example: How much heat is required to raise the temperature of a 25-g aluminum cube from 60°C to 80°C? Note that the temperature change is ∆T = 80°C – 60°C = 20°C. Looking up the value of the specific heat of aluminum in the text, we find Q = mc∆T = 25 g × 0.215 cal / g°C × 20°C = 108 cal Practice: ...
... Example: How much heat is required to raise the temperature of a 25-g aluminum cube from 60°C to 80°C? Note that the temperature change is ∆T = 80°C – 60°C = 20°C. Looking up the value of the specific heat of aluminum in the text, we find Q = mc∆T = 25 g × 0.215 cal / g°C × 20°C = 108 cal Practice: ...
• Heating foods • Moist-heat method • Dry
... • The muscle portion of most meat, poultry, and fish is composed of 75% water and 20% protein. The ability of these items to hold water and contain fat affects their juiciness. • Collagen, an important protein found in meat and poultry, forms the basic structure of connective tissue. It is the struc ...
... • The muscle portion of most meat, poultry, and fish is composed of 75% water and 20% protein. The ability of these items to hold water and contain fat affects their juiciness. • Collagen, an important protein found in meat and poultry, forms the basic structure of connective tissue. It is the struc ...
Science Unit 5 Powerpoint 2 Energy
... For example, suppose you place an ice cube in a glass of water. Because the water is warmer than the ice, heat flows from the water to the ice until the two reach the same temperature. Heat does not flow from the ice to the water. ...
... For example, suppose you place an ice cube in a glass of water. Because the water is warmer than the ice, heat flows from the water to the ice until the two reach the same temperature. Heat does not flow from the ice to the water. ...
EF - hrsbstaff.ednet.ns.ca
... Ellen Fraser Study Guide: Chapter 6 Energy and Chemical Change What is thermochemistry? Thermochemistry is a branch of chemistry, which deals with energy that is absorbed or released during a chemical reaction. Heat (thermal energy) can be transferred between objects having different temperatures. W ...
... Ellen Fraser Study Guide: Chapter 6 Energy and Chemical Change What is thermochemistry? Thermochemistry is a branch of chemistry, which deals with energy that is absorbed or released during a chemical reaction. Heat (thermal energy) can be transferred between objects having different temperatures. W ...
Unit 6 Review
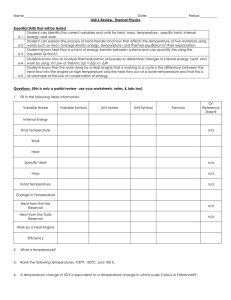
... Essential Skills that will be tested Student can identify the correct variables and units for heat, mass, temperature, specific heat, internal 6-1 energy, and work. Student can explain the process of heat transfer and how that affects the temperature of two materials using 6-2 words such as heat, av ...
... Essential Skills that will be tested Student can identify the correct variables and units for heat, mass, temperature, specific heat, internal 6-1 energy, and work. Student can explain the process of heat transfer and how that affects the temperature of two materials using 6-2 words such as heat, av ...
Review of 17.1, 17.2 and 17.3 Name: 1.) When 2 moles of NO burn
... determine the specific heat capacity of brick. Based on the evidence obtained in this experiment, 16 kJ of energy was transferred to a 938 g brick as the temperature of the brick changed 19.5 oC to 35.0oC. Calculate the specific heat capacity of the brick. ...
... determine the specific heat capacity of brick. Based on the evidence obtained in this experiment, 16 kJ of energy was transferred to a 938 g brick as the temperature of the brick changed 19.5 oC to 35.0oC. Calculate the specific heat capacity of the brick. ...
Introduction - UniMAP Portal
... Calculate the convection heat transfer through forced convection heat transfer inside pipe (dimensionless no.), heat transfer coefficient for laminar flow inside pipe, heat transfer coefficient for turbulent flow inside pipe, heat transfer coefficient for transition flow inside pipe, heat transfer c ...
... Calculate the convection heat transfer through forced convection heat transfer inside pipe (dimensionless no.), heat transfer coefficient for laminar flow inside pipe, heat transfer coefficient for turbulent flow inside pipe, heat transfer coefficient for transition flow inside pipe, heat transfer c ...
Ch. 5: Thermochemistry
... Heat capacity = amount of heat to raise temp by 1K (or 1º C) Specific heat = heat capacity for 1 g of a substance (Symbol for specific heat is usually C) ...
... Heat capacity = amount of heat to raise temp by 1K (or 1º C) Specific heat = heat capacity for 1 g of a substance (Symbol for specific heat is usually C) ...
Cold Plate - L.D.S. System
... The use of water-cooled heat sinks is becoming increasingly common in response to the need of dissipating high power in small spaces and without the use of fans with high air flow. In fact, the water, in virtue of a specific heat much higher than air, it is far more effective as a vector fluid in re ...
... The use of water-cooled heat sinks is becoming increasingly common in response to the need of dissipating high power in small spaces and without the use of fans with high air flow. In fact, the water, in virtue of a specific heat much higher than air, it is far more effective as a vector fluid in re ...
Done by: Terence Lee (27) - ScienceIMPORTANTRCYJTLCEC
... for a long period and thus can keep the interior of the container either hot or cold. Since corrugated cardboard is made up of two layers of paper with fluting in between, air is trapped within the cardboard, thus providing good insulation, due to the properties of air as said above. Cork Our group ...
... for a long period and thus can keep the interior of the container either hot or cold. Since corrugated cardboard is made up of two layers of paper with fluting in between, air is trapped within the cardboard, thus providing good insulation, due to the properties of air as said above. Cork Our group ...
performances of flat-plate and cpc solar collectors in underfloor
... The objective of this study is to investigate the possibility of using Compound Parabolic Collector (CPC) collectors to replace Flat-Plat collectors in solar energy underfloor heating systems. By this way, it is aimed to explore the feasibility of area reduction required by the collectors. Secondly, ...
... The objective of this study is to investigate the possibility of using Compound Parabolic Collector (CPC) collectors to replace Flat-Plat collectors in solar energy underfloor heating systems. By this way, it is aimed to explore the feasibility of area reduction required by the collectors. Secondly, ...
Heat Energy and Temperature Notes
... move more vigorously. - When a substance loses heat its molecular action decreases. - Absolute Zero is the point at which all molecular motion would stop. (no heat left!!) ...
... move more vigorously. - When a substance loses heat its molecular action decreases. - Absolute Zero is the point at which all molecular motion would stop. (no heat left!!) ...
Honors Chemistry Quiz Chapter 6: Thermochemistry - Doc-U-Ment
... 1) Energy that is associated with the position or composition of an object is called A) kinetic energy B) thermal energy C) potential energy D) chemical energy E) None of the above. 2) Which of the following signs on q and w represent a system that is doing work on the surroundings, as well as losin ...
... 1) Energy that is associated with the position or composition of an object is called A) kinetic energy B) thermal energy C) potential energy D) chemical energy E) None of the above. 2) Which of the following signs on q and w represent a system that is doing work on the surroundings, as well as losin ...
ABE 484
... methods. Learn how to interpret the data for the specific need of each problem. Promote critical thinking through problem solving processes. 4. Learn how to set up governing equations, boundary conditions, and initial conditions under various circumstances with different geometries. 5. Use requisite ...
... methods. Learn how to interpret the data for the specific need of each problem. Promote critical thinking through problem solving processes. 4. Learn how to set up governing equations, boundary conditions, and initial conditions under various circumstances with different geometries. 5. Use requisite ...
Exercises – Chapter 8
... E.18 The hotter the water, the more thermal energy can be harnessed and the greater the temperature difference between the hot water and the colder surrounding, the more efficient the hurricane is at converting that thermal energy into mechanical work. 19. On a clear sunny day, the ground is heated ...
... E.18 The hotter the water, the more thermal energy can be harnessed and the greater the temperature difference between the hot water and the colder surrounding, the more efficient the hurricane is at converting that thermal energy into mechanical work. 19. On a clear sunny day, the ground is heated ...
Ideal Gas Law / Heat Transfer
... Good conductors are better at transferring heat when touching other molecules (many metals) ...
... Good conductors are better at transferring heat when touching other molecules (many metals) ...
Examination Heat Transfer
... calculate then Nu L and express Nu L in Nu L (Nusselt at position x = L). c) Water at the rate of 68 kg/min is heated from 35 to 75 0C by an oil having a specific heat of 1.9 kJ/kg.0C. The oil enters the exchanger at 110 0C and leaves at 75 0C. The overall heat transfer coefficient is 320 W/m2.K. Th ...
... calculate then Nu L and express Nu L in Nu L (Nusselt at position x = L). c) Water at the rate of 68 kg/min is heated from 35 to 75 0C by an oil having a specific heat of 1.9 kJ/kg.0C. The oil enters the exchanger at 110 0C and leaves at 75 0C. The overall heat transfer coefficient is 320 W/m2.K. Th ...
doc - University of Colorado Boulder
... plant hormone), can have large effects on plant growth and have to be controlled. This can be accomplished using a ______________. At night, low light, or during germination, plants produce _____ (gas) and need ______ (gas) for their metabolism. In order to not exceed toxic levels of this gas in the ...
... plant hormone), can have large effects on plant growth and have to be controlled. This can be accomplished using a ______________. At night, low light, or during germination, plants produce _____ (gas) and need ______ (gas) for their metabolism. In order to not exceed toxic levels of this gas in the ...
The increasing number of heat pumps is not growing the peak
... Heat pumps decrease the total energy consumption on an annual basis but might increase the momentarily electric power demand during the heating season, if the heat pumps replace other heating sources than electric heating. On the other hand, by using heat pumps to replace electric heating, the power ...
... Heat pumps decrease the total energy consumption on an annual basis but might increase the momentarily electric power demand during the heating season, if the heat pumps replace other heating sources than electric heating. On the other hand, by using heat pumps to replace electric heating, the power ...
CHAPTER 14 Energy in the Atmosphere
... • Transfer of heat through DIRECT CONTACT • Energy passed from fast-moving molecules (HOT) to slower-moving molecules (COOLER) • Does not work well in liquids or gases • Examples: Walking barefoot on hot sand ...
... • Transfer of heat through DIRECT CONTACT • Energy passed from fast-moving molecules (HOT) to slower-moving molecules (COOLER) • Does not work well in liquids or gases • Examples: Walking barefoot on hot sand ...