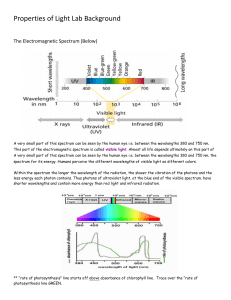
What Are Electromagnetic Waves?
... 0.001-10 nm (about the size of an atom). As the wavelengths of light decrease, they increase in energy. Xrays have smaller wavelengths and therefore higher energy than ultraviolet waves. We usually talk about X-rays in terms of their energy rather than wavelength. This is partially because X-rays ha ...
... 0.001-10 nm (about the size of an atom). As the wavelengths of light decrease, they increase in energy. Xrays have smaller wavelengths and therefore higher energy than ultraviolet waves. We usually talk about X-rays in terms of their energy rather than wavelength. This is partially because X-rays ha ...
Human exposure to Electromagnetic Fields
... Radio waves have lower frequencies and longer wavelengths than microwaves. National television and radio programs are broadcast using radio waves. Radio waves are defracted around hills and buildings so there is less chance of a “shadow” causing poor reception. Radio waves have a low frequency which ...
... Radio waves have lower frequencies and longer wavelengths than microwaves. National television and radio programs are broadcast using radio waves. Radio waves are defracted around hills and buildings so there is less chance of a “shadow” causing poor reception. Radio waves have a low frequency which ...
Electromagnetic Spectrum
... When the sun emits radiation with frequencies in the range of visible light, this radiation is viewable by humans Light can cause chemical reactions like photosynthesis. ...
... When the sun emits radiation with frequencies in the range of visible light, this radiation is viewable by humans Light can cause chemical reactions like photosynthesis. ...
Ultraviolet

Ultraviolet (UV) light is an electromagnetic radiation with a wavelength from 400 nm to 100 nm, shorter than that of visible light but longer than X-rays. Though usually invisible, under some conditions children and young adults can see ultraviolet down to wavelengths of about 310 nm, and people with aphakia (missing lens) can also see some UV wavelengths. Near-UV is visible to a number of insects and birds.UV radiation is present in sunlight, and is produced by electric arcs and specialized lights such as mercury-vapor lamps, tanning lamps, and black lights. Although lacking the energy to ionize atoms, long-wavelength ultraviolet radiation can cause chemical reactions, and causes many substances to glow or fluoresce. Consequently, biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules.Suntan, freckling and sunburn are familiar effects of over-exposure, along with higher risk of skin cancer. Living things on dry land would be severely damaged by ultraviolet radiation from the sun if most of it were not filtered out by the Earth's atmosphere, particularly the ozone layer. More-energetic, shorter-wavelength ""extreme"" UV below 121 nm ionizes air so strongly that it is absorbed before it reaches the ground.Ultraviolet is also responsible for the formation of bone-strengthening vitamin D in most land vertebrates, including humans. The UV spectrum thus has effects both beneficial and harmful to human health.


