Nuclear - chemmybear.com
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
NUCLEAR CHEMISTRY
... intermediate mass 2. The mass of the products is less than the mass of the reactants. Missing mass is converted to energy a. Small amounts of missing mass are converted to HUGE amounts of energy (E = mc2) ...
... intermediate mass 2. The mass of the products is less than the mass of the reactants. Missing mass is converted to energy a. Small amounts of missing mass are converted to HUGE amounts of energy (E = mc2) ...
NUCLEAR FISSION- a Tunneling Process NUCLEAR FUSION
... chain reaction. All this was worked out by Frisch & Meitner within days of hearing of Neutron-induced fission- with accompanying the discovery of fission. emission of 2 neutrons ...
... chain reaction. All this was worked out by Frisch & Meitner within days of hearing of Neutron-induced fission- with accompanying the discovery of fission. emission of 2 neutrons ...
Nuclear Physics - fission, fusion, and the stars
... chain reaction. All this was worked out by Frisch & Meitner within days of hearing of Neutron-induced fission- with accompanying the discovery of fission. emission of 2 neutrons ...
... chain reaction. All this was worked out by Frisch & Meitner within days of hearing of Neutron-induced fission- with accompanying the discovery of fission. emission of 2 neutrons ...
Chapter 7 - Bakersfield College
... D. Binding energy makes stable heavier nuclei possible (beyond hydrogen) which in turn accounts for the various elements and forms of matter found in the physical universe. 7-9. Nuclear Fission A. In 1939, uranium-235 was discovered to undergo nuclear fission when struck by a neutron. 1. A nucleus o ...
... D. Binding energy makes stable heavier nuclei possible (beyond hydrogen) which in turn accounts for the various elements and forms of matter found in the physical universe. 7-9. Nuclear Fission A. In 1939, uranium-235 was discovered to undergo nuclear fission when struck by a neutron. 1. A nucleus o ...
1.3. Basic Principles of Nuclear Physics
... produces mainly 20N also: 12C(12C, p)23Na & 12C(12C, n)23Mg ...
... produces mainly 20N also: 12C(12C, p)23Na & 12C(12C, n)23Mg ...
Adobe Acrobat file ()
... Binding Energy Notes Except for light nuclei, the binding energy is about 8 MeV per nucleon The curve peaks in the vicinity of A = 60 ...
... Binding Energy Notes Except for light nuclei, the binding energy is about 8 MeV per nucleon The curve peaks in the vicinity of A = 60 ...
Beta-delayed two-neutron emission
... emission is observed. Even further away, -delayed two-nucleon emission can be observed and studied. -delayed two-proton emission has been observed experimentally for 9 different nuclei, but only the decay of 31Ar has been studied to some extent. On the neutron-rich side, b-delayed two-neutron emis ...
... emission is observed. Even further away, -delayed two-nucleon emission can be observed and studied. -delayed two-proton emission has been observed experimentally for 9 different nuclei, but only the decay of 31Ar has been studied to some extent. On the neutron-rich side, b-delayed two-neutron emis ...
sep04 neutrinos - Charles J Horowitz
... energy. Namely the possibility that there exists in nuclei electrically neutral particles, that I call neutrons, which have spin ½… The mass of the neutrons should be of the same order as the electron mass and in any event not larger than 0.01 proton masses. The continuous beta spectrum would then b ...
... energy. Namely the possibility that there exists in nuclei electrically neutral particles, that I call neutrons, which have spin ½… The mass of the neutrons should be of the same order as the electron mass and in any event not larger than 0.01 proton masses. The continuous beta spectrum would then b ...
isotope - Aurora City Schools
... only ½ the original to remain (and ½ to decay) • It decays into another isotope of the same element, or into another element. It doesn’t just disappear. ...
... only ½ the original to remain (and ½ to decay) • It decays into another isotope of the same element, or into another element. It doesn’t just disappear. ...
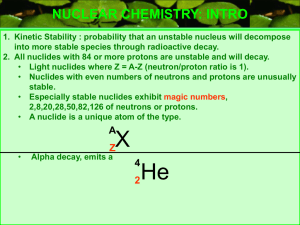
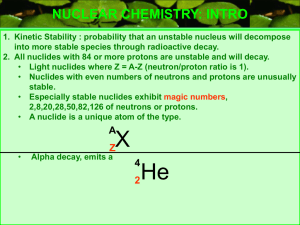
NUCLEAR CHEMISTRY: INTRO
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
radioactive decay - Aurora City Schools
... only ½ the original to remain (and ½ to decay) • It decays into another isotope of the same element, or into another element. It doesn’t just disappear. ...
... only ½ the original to remain (and ½ to decay) • It decays into another isotope of the same element, or into another element. It doesn’t just disappear. ...
1 AP Chemistry Chapter 21 - The Nucleus: A Chemist`s View 21.1
... C → 147 N + −10 β (1) Living things take in carbon-12 and carbon-14, in a fixed ratio (2) When a living thing dies, the amount of carbon-12 does not change, but carbon-14 begins to decrease through decay ...
... C → 147 N + −10 β (1) Living things take in carbon-12 and carbon-14, in a fixed ratio (2) When a living thing dies, the amount of carbon-12 does not change, but carbon-14 begins to decrease through decay ...
Chapter 32 Nuclear Physics
... each of its constitutes (protons and neutrons) is about 1800 times more massive than an electron. We will see that many nuclei, particularly those of the heavier elements, are unstable and spontaneous break apart, or disintegrate, into other nuclei. This spontaneous disintegration is called radioact ...
... each of its constitutes (protons and neutrons) is about 1800 times more massive than an electron. We will see that many nuclei, particularly those of the heavier elements, are unstable and spontaneous break apart, or disintegrate, into other nuclei. This spontaneous disintegration is called radioact ...
Ch 21.1 Study Guide
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
Nuclear force
... • if the approaching nucleon is a neutron, things are a lot easier, because the potential well will be in a “flat surface”. It doesn’t matter if it is approaching a proton, because it will not feel its electrostatic field. • However, if the neutron is too fast (high KE), it will go past the potentia ...
... • if the approaching nucleon is a neutron, things are a lot easier, because the potential well will be in a “flat surface”. It doesn’t matter if it is approaching a proton, because it will not feel its electrostatic field. • However, if the neutron is too fast (high KE), it will go past the potentia ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.