Radioactivity - Williamstown Independent Schools
... the more stable the nucleus. • This binding energy is reported per nucleon. ...
... the more stable the nucleus. • This binding energy is reported per nucleon. ...
Basic properties of atomic nuclei
... (a) What is the energy difference between the states with the nuclear spin angular momentum components parallel and anti parallel to the field? Which state is lower in energy, the one with its spin component parallel to the fleld or the one with its spin component antiparallel to the field? How do y ...
... (a) What is the energy difference between the states with the nuclear spin angular momentum components parallel and anti parallel to the field? Which state is lower in energy, the one with its spin component parallel to the fleld or the one with its spin component antiparallel to the field? How do y ...
Modern Physics TEST
... ____ 11. If the stable nuclei are plotted with neutron number versus proton number, the curve formed by the stable nuclei does not follow the line N = Z. Which of the following influences the binding energy so that this “valley of stability” forms? (see plot right) a. the volume of the nucleus c. th ...
... ____ 11. If the stable nuclei are plotted with neutron number versus proton number, the curve formed by the stable nuclei does not follow the line N = Z. Which of the following influences the binding energy so that this “valley of stability” forms? (see plot right) a. the volume of the nucleus c. th ...
Energy Production in Stars
... The conversion of mass to energy accounts for the enormous energy output of the stars What physical mechanisms can cause this? Nuclear fission - splitting of an atom's nucleus Nuclear fusion - sticking two nuclei together Nuclear fusion is favored because: o The most stable nuclei in the u ...
... The conversion of mass to energy accounts for the enormous energy output of the stars What physical mechanisms can cause this? Nuclear fission - splitting of an atom's nucleus Nuclear fusion - sticking two nuclei together Nuclear fusion is favored because: o The most stable nuclei in the u ...
CH2ch19_1
... a) Protons = +1 charge, 1 mass unit (Atomic Number = Z = # of protons) b) Neutrons = 0 charge, 1 mass unit c) Mass Number = A = sum of neutrons + protons d) Isotopes = same atomic number but different mass numbers (#’s of neutrons) e) Nuclide = a particular isotope A Z ...
... a) Protons = +1 charge, 1 mass unit (Atomic Number = Z = # of protons) b) Neutrons = 0 charge, 1 mass unit c) Mass Number = A = sum of neutrons + protons d) Isotopes = same atomic number but different mass numbers (#’s of neutrons) e) Nuclide = a particular isotope A Z ...
Energy per nucleon
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . ...
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . ...
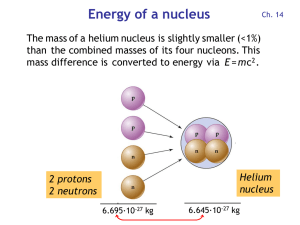
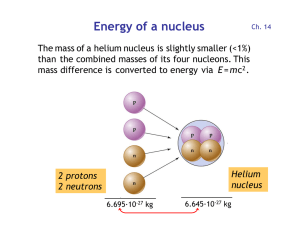
Energy of a nucleus
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nucle ...
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nucle ...
2.10 Basic Nuclear Chemistry
... C. Beta Emission (β) or (0-1β) 1. This is when a neutron is converted to a proton and an electron. The electron is emitted from the nucleus. 2. The Atomic Number increases by 1, but the Atomic Mass remains the same in AMUs. 3. This has greater penetrating strength than Alpha particle emission. D. Po ...
... C. Beta Emission (β) or (0-1β) 1. This is when a neutron is converted to a proton and an electron. The electron is emitted from the nucleus. 2. The Atomic Number increases by 1, but the Atomic Mass remains the same in AMUs. 3. This has greater penetrating strength than Alpha particle emission. D. Po ...
Stellar energy - schoolphysics
... Note: the apparent imbalance between particles in these equations can be understood if we look at the first of these. On the left we have two protons and no neutrons. On the right we have one proton and one neutron and a positron. One of the protons has been converted into a neutron and a positron. ...
... Note: the apparent imbalance between particles in these equations can be understood if we look at the first of these. On the left we have two protons and no neutrons. On the right we have one proton and one neutron and a positron. One of the protons has been converted into a neutron and a positron. ...
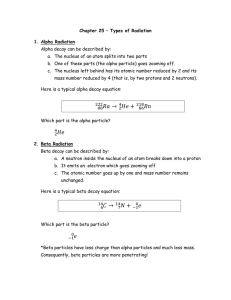
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
Nuclear Physics SL - Hockerill Students
... Fission: Fission is the name given to the nuclear reaction whereby large nuclei are induced to break up into smaller nuclei and release energy in the process. It is the reaction that is used in nuclear reactors and atomic bombs. A typical single reaction might involve bombarding a uranium nucleus ...
... Fission: Fission is the name given to the nuclear reaction whereby large nuclei are induced to break up into smaller nuclei and release energy in the process. It is the reaction that is used in nuclear reactors and atomic bombs. A typical single reaction might involve bombarding a uranium nucleus ...
Example 27-3 The Binding Energy of 4He
... binding energy of the nucleus divided by the number of nucleons in the nucleus. ...
... binding energy of the nucleus divided by the number of nucleons in the nucleus. ...
Quantum Tunneling
... never to return. An example is nuclear fission, possible in heavy nuclei of plutonium or uranium235. Their nucleus contains so many protons trying to push it apart (with their electric repulsion), that adding just a modest amount energy--released when an extra neutron is allowed to be pulled into th ...
... never to return. An example is nuclear fission, possible in heavy nuclei of plutonium or uranium235. Their nucleus contains so many protons trying to push it apart (with their electric repulsion), that adding just a modest amount energy--released when an extra neutron is allowed to be pulled into th ...
2005 Nuclear FRQs - AP Chemistry Olympics
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
Nuclear drip line

In nuclear physics, the boundaries for nuclear particle-stability are called drip lines. Atomic nuclei contain both protons and neutrons—the number of protons defines the identity of that element (ie, carbon always has 6 protons), but the number of neutrons within that element may vary (carbon-12 and its isotope carbon-13, for example). The number of isotopes each element may have is visually represented by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa (X axis) and number of protons increasing along the ordinate (Y axis). The resulting chart is commonly referred to as the table of nuclides, and is to nuclear physics what the periodic table of the elements is to chemistry.An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the nuclear chart by adding one type of nucleon (i.e. a proton or neutron, both called nucleons) to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has 'leaked' or 'dripped' out of the nucleus, hence giving rise to the term ""drip line"". Drip lines are defined for protons, neutrons, and alpha particles, and these all play important roles in nuclear physics. The nucleon drip lines are at the extreme of the proton-to-neutron ratio: at p:n ratios at or beyond the driplines, no stable nuclei can exist. The location of the neutron drip line is not well known for most of the nuclear chart, whereas the proton and alpha driplines have been measured for a wide range of elements. The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: in the water case, there is a lower potential available that is great enough to overcome surface tension and so produces a droplet; in the case of nuclei, the emission of a particle from a nucleus, against the strong nuclear force, leaves the total potential of the nucleus and the emitted particle in a lower state. Because nucleons are quantized, only integer values are plotted on the table of isotopes; this indicates that the drip line is not linear but instead looks like a step function up close.