Chemistry 354 - Homework Set IV
... combustion of a single molecule of sucrose; the distance from sideline to sideline on a football field; the time required for an electron to make one circuit of the nucleus in the Bohr atom; the mass of a hydrogen atom; the mass of Haystacks Calhoun (erstwhile professional wrestler); the distance be ...
... combustion of a single molecule of sucrose; the distance from sideline to sideline on a football field; the time required for an electron to make one circuit of the nucleus in the Bohr atom; the mass of a hydrogen atom; the mass of Haystacks Calhoun (erstwhile professional wrestler); the distance be ...
PowerPoint - OrgSites.com
... Confused??? You’ve Got Company! “No familiar conceptions can be woven around the electron; something unknown is doing we don’t know what.” Physicist Sir Arthur Eddington The Nature of the Physical World ...
... Confused??? You’ve Got Company! “No familiar conceptions can be woven around the electron; something unknown is doing we don’t know what.” Physicist Sir Arthur Eddington The Nature of the Physical World ...
Introduction to Quantum theory, and the
... case, then if the intensity of the light were low, no ejection of electrons would be observed, or a certain amount of time at least should pass until an electron has acquired enough energy to be emitted. However, as can be seen experimental observations contradicted these assumptions. Also if the li ...
... case, then if the intensity of the light were low, no ejection of electrons would be observed, or a certain amount of time at least should pass until an electron has acquired enough energy to be emitted. However, as can be seen experimental observations contradicted these assumptions. Also if the li ...
NATURAL UNITS AND PLANE WAVES Natural Units A.1
... have developed the knack of placing the constants back into the final answers, dimensional analysis is still possible. The basis of our natural units has Planck’s constant h = 1 and the speed of light c = 1. To convert to natural units just take your formulas in conventional units and set h = 1 and ...
... have developed the knack of placing the constants back into the final answers, dimensional analysis is still possible. The basis of our natural units has Planck’s constant h = 1 and the speed of light c = 1. To convert to natural units just take your formulas in conventional units and set h = 1 and ...
Chapter 5 reveiw
... 5. Be able to identify the correct no. of valance electrons outer energy level in any atom. a. EX: in Sulfur there are __________ valence electrons 6. Be able to identify the number of unpaired electrons or half filled orbitals in any atom. a. Ex: in Oxygen there are ______ half filled orbitals 7. E ...
... 5. Be able to identify the correct no. of valance electrons outer energy level in any atom. a. EX: in Sulfur there are __________ valence electrons 6. Be able to identify the number of unpaired electrons or half filled orbitals in any atom. a. Ex: in Oxygen there are ______ half filled orbitals 7. E ...
Conjugated Bonding in Cyanine Dyes: A "Particle In A Box" Model
... According to Kuhn, the length of the conjugated chain should be taken as "the length of the polymethine ziz-zag chain between the nitrogen atoms plus one bond distance to either side." The purpose of including a "bond distance" on either side of the Nitrogen atoms is to include the "distance" occupi ...
... According to Kuhn, the length of the conjugated chain should be taken as "the length of the polymethine ziz-zag chain between the nitrogen atoms plus one bond distance to either side." The purpose of including a "bond distance" on either side of the Nitrogen atoms is to include the "distance" occupi ...
A Primer on Quantum Mechanics and Orbitals
... What is also true is that this function k is also an eigenfunction of the Hamiltonian. Remember the Hamilton operator is composed of two parts, the kinetic energy operator and the potential energy operator. The Hamiltonian operator can then be seen as the operator, i.e. mathematical procedure, that ...
... What is also true is that this function k is also an eigenfunction of the Hamiltonian. Remember the Hamilton operator is composed of two parts, the kinetic energy operator and the potential energy operator. The Hamiltonian operator can then be seen as the operator, i.e. mathematical procedure, that ...
atomsagain
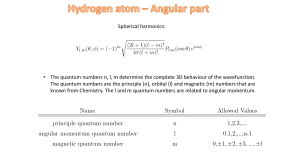
... n,l ,m r, , Rn,l r Yl .m , The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the stat ...
... n,l ,m r, , Rn,l r Yl .m , The full wave function is given by: •n, the principal quantum number, tells you the energy n 1, 2,3, •l tells you the total angular momentum l 0,1, 2, , n 1 •m tells you the angular momentum around the z-axis An electron in hydrogen is in the stat ...
슬라이드 1
... small flask of hydrocyanic acid. If one has left this entire system to itself for an hour, one would say that the cat still lives if meanwhile no atom has decayed. The psi-function of the entire system would express this by having in it the living and dead cat (pardon the expression) mixed or smeare ...
... small flask of hydrocyanic acid. If one has left this entire system to itself for an hour, one would say that the cat still lives if meanwhile no atom has decayed. The psi-function of the entire system would express this by having in it the living and dead cat (pardon the expression) mixed or smeare ...
The Quantum Mechanical Model and Electron
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
CH 27 – Quantum Physics
... effects. The particle-like properties are demonstrated in phenomena in which the concept of the photon is important, such as the photoelectric effect and the Compton effect. For the photon, the energy and momentum relationships are ...
... effects. The particle-like properties are demonstrated in phenomena in which the concept of the photon is important, such as the photoelectric effect and the Compton effect. For the photon, the energy and momentum relationships are ...
chapter 7 quiz
... 3.__I__Particle with no charge. C) Ernest Rutherford 4.__Q__Invented the gas discharge tube. D) anode 5.__L__Shoot electrons in a gas discharge tube. E) Harry Potter 6.__D__Electrons are shot towards it in a gas discharge F) television tube. G) atomic number 7.__S__Arranged the periodic table using ...
... 3.__I__Particle with no charge. C) Ernest Rutherford 4.__Q__Invented the gas discharge tube. D) anode 5.__L__Shoot electrons in a gas discharge tube. E) Harry Potter 6.__D__Electrons are shot towards it in a gas discharge F) television tube. G) atomic number 7.__S__Arranged the periodic table using ...