cuantica
... in the computation tree is obtained by multiplying the probability amplitudes on that path. In the example, the red path has amplitude 1/2, and the green path has amplitude –1/2. The probability amplitude for getting the answer |0 is obtained by adding the probability amplitudes… notice that the ph ...
... in the computation tree is obtained by multiplying the probability amplitudes on that path. In the example, the red path has amplitude 1/2, and the green path has amplitude –1/2. The probability amplitude for getting the answer |0 is obtained by adding the probability amplitudes… notice that the ph ...
Chapter 4 Electron Configuration
... substance that matches the frequency of the wave, the substance will be transparent to that wave. Communication waves – electrons pulsing back and forth along a wire Visible and ultraviolet – electrons being excited to higher energy levels ...
... substance that matches the frequency of the wave, the substance will be transparent to that wave. Communication waves – electrons pulsing back and forth along a wire Visible and ultraviolet – electrons being excited to higher energy levels ...
Problem set 11
... 1. h9i Consider force free motion of a symmetric top with I1 = I2 , as discussed in the lecture. Suppose the axis of the top makes an angle θ , 0 with the fixed direction of L. (a) h6i Find the angle α between the angular velocity vector Ω and angular momentum vector L (α is half the opening angle o ...
... 1. h9i Consider force free motion of a symmetric top with I1 = I2 , as discussed in the lecture. Suppose the axis of the top makes an angle θ , 0 with the fixed direction of L. (a) h6i Find the angle α between the angular velocity vector Ω and angular momentum vector L (α is half the opening angle o ...
Epistemological Foun.. - University of Manitoba
... It was hard to see why, after one once knew precisely the position and velocity of a particle, its future could not be determined exactly due to the disturbance of an object by the act of observing it. […] Thinking in this vein, he had the key insight into the origins of the indeterminacy at the ato ...
... It was hard to see why, after one once knew precisely the position and velocity of a particle, its future could not be determined exactly due to the disturbance of an object by the act of observing it. […] Thinking in this vein, he had the key insight into the origins of the indeterminacy at the ato ...
Chapter 7
... • The Rutherford model could not explain these results, but Bohr’s “planetary” or quantum model could (1914). • Bohr assumed quantized orbital angular momentum values such that when centrifugal force out (merry-go-round) = electrostatic attraction in, the electron was in a stable state. • This model ...
... • The Rutherford model could not explain these results, but Bohr’s “planetary” or quantum model could (1914). • Bohr assumed quantized orbital angular momentum values such that when centrifugal force out (merry-go-round) = electrostatic attraction in, the electron was in a stable state. • This model ...
Chapter 1 Quiz
... 3.(20 pts) A box of dimensions Lx = 20nm, Ly=15nm, and Lz=50nm has walls which can be treated as infinite potential barriers. 5 electrons are placed in the box. ...
... 3.(20 pts) A box of dimensions Lx = 20nm, Ly=15nm, and Lz=50nm has walls which can be treated as infinite potential barriers. 5 electrons are placed in the box. ...
Chapter 9 review
... If molecules, atoms, or subatomic particles are in the liquid or solid state, the Pauli exclusion principle prevents two particles with identical wave functions from sharing the same space. ...
... If molecules, atoms, or subatomic particles are in the liquid or solid state, the Pauli exclusion principle prevents two particles with identical wave functions from sharing the same space. ...
The statistical interpretation of quantum mechanics
... matrix by the general concept of an operator, and thus made it possible to describe aperiodic processes. Nevertheless we missed the correct approach. This was left to Schrödinger, and I immediately took up his method since it held promise of leading to an interpretation of the ψ-function. Again an i ...
... matrix by the general concept of an operator, and thus made it possible to describe aperiodic processes. Nevertheless we missed the correct approach. This was left to Schrödinger, and I immediately took up his method since it held promise of leading to an interpretation of the ψ-function. Again an i ...
Light and the electron
... that electromagnetic radiation has both wavelike and particle-like natures. ► Extended upon Planck’s equation – photoelectric effect ► Pg. 124 practice problems 5,6 ...
... that electromagnetic radiation has both wavelike and particle-like natures. ► Extended upon Planck’s equation – photoelectric effect ► Pg. 124 practice problems 5,6 ...
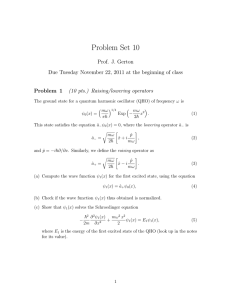
Problem Set 10
... The energy of each particle is E > V0 . The particles are initially moving to the right, coming from x = −∞. (a) Write down the wave function for x < 0. Here, are there left- and right-moving components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and r ...
... The energy of each particle is E > V0 . The particles are initially moving to the right, coming from x = −∞. (a) Write down the wave function for x < 0. Here, are there left- and right-moving components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and r ...
Introduction to Quantum Systems
... Elements of quantum physics: Quantization of physical magnitudes Spin Stationary states Indistinguishable particle systems Quantum systems with electromagnetic interaction (atoms, molecules and solids): The hydrogen atom: electronic configuration Interaction of atomic systems with light: lasers The ...
... Elements of quantum physics: Quantization of physical magnitudes Spin Stationary states Indistinguishable particle systems Quantum systems with electromagnetic interaction (atoms, molecules and solids): The hydrogen atom: electronic configuration Interaction of atomic systems with light: lasers The ...
l = 0
... Translation from Spectroscopic Notation to Quantum numbers For larger atom the assignment of quantum numbers must continue following the rules until the number of electrons corresponding to the particular atom is reached. Writing quantum number for a particular electron can be made easier by transl ...
... Translation from Spectroscopic Notation to Quantum numbers For larger atom the assignment of quantum numbers must continue following the rules until the number of electrons corresponding to the particular atom is reached. Writing quantum number for a particular electron can be made easier by transl ...
Homework 8
... Find the hamiltonian, H for a mass m confined to the x axis and subject to a force F = −kx3 where k > 0. Sketch and describe the phase-space orbits. A beam of protons is moving along an accelerator pipe in the z-direction. The particles are uniformly distributed in a cylindrical volume of length L0 ...
... Find the hamiltonian, H for a mass m confined to the x axis and subject to a force F = −kx3 where k > 0. Sketch and describe the phase-space orbits. A beam of protons is moving along an accelerator pipe in the z-direction. The particles are uniformly distributed in a cylindrical volume of length L0 ...
Particles & Strings - University of Southampton
... Quantum Gravity If the vacuum is full of all this stuff shouldn’t we be pulled gravitationally by it? Since it is uniformily distributed there is no net pull (equal space to all sides) But General Relativity says the energy should uniformily curve space-time… the Universe should be the size of a gr ...
... Quantum Gravity If the vacuum is full of all this stuff shouldn’t we be pulled gravitationally by it? Since it is uniformily distributed there is no net pull (equal space to all sides) But General Relativity says the energy should uniformily curve space-time… the Universe should be the size of a gr ...
The Learnability of Quantum States
... So why aren’t we done? Because real quantum experiments are subject to noise Would an efficient classical algorithm that simulated a noisy optics experiment still collapse the polynomial hierarchy? Main Result: Yes, assuming two plausible conjectures about permanents of random matrices (the “PCC” a ...
... So why aren’t we done? Because real quantum experiments are subject to noise Would an efficient classical algorithm that simulated a noisy optics experiment still collapse the polynomial hierarchy? Main Result: Yes, assuming two plausible conjectures about permanents of random matrices (the “PCC” a ...
Unit 3 Study Guide
... Proposed the Uncertainty principle – the electron is only probably located in orbital Proposed electrons and other particles have wavelengths Developed the first periodic table; Predicted the discovery of Gallium ...
... Proposed the Uncertainty principle – the electron is only probably located in orbital Proposed electrons and other particles have wavelengths Developed the first periodic table; Predicted the discovery of Gallium ...